Abstract
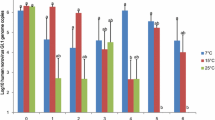
Seasonally recurrent outbreaks of mass mortality in Pacific oysters (Crassostrea gigas) caused by microvariant genotypes of ostreid herpesvirus 1 (OsHV-1) occur in Europe, New Zealand and Australia. The incubation period for OsHV-1 under experimental conditions is 48-72 hours and depends on water temperature, as does the mortality. An in vivo growth curve for OsHV-1 was determined by quantifying OsHV-1 DNA at 10 time points between 2 and 72 hours after exposure to OsHV-1. The peak replication rate was the same at 18 °C and 22 °C; however, there was a longer period of amplification leading to a higher peak concentration at 22 °C (2.34 × 107 copies/mg at 18 hours) compared to 18 °C (1.38 × 105 copies/mg at 12 hours). The peak viral concentration preceded mortality by 72 hours and 20 hours at 18 °C and 22 °C, respectively. Cumulative mortality to day 14 was 45.9% at 22 °C compared to 0.3% at 18 °C. The prevalence of OsHV-1 infection after 14 days at 18 °C was 33.3%. No mortality from OsHV-1 occurred when the water temperature in tanks of oysters challenged at 18 °C was increased to 22 °C for 14 days. The influence of water temperature prior to exposure to OsHV-1 and during the initial virus replication is an important determinant of the outcome of infection in C. gigas.


Similar content being viewed by others
References
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 1(1)
de Kantzow M, Hick P, Becker JA, Whittington RJ (2016) Effect of water temperature on mortality of Pacific oysters Crassostrea gigas associated with microvariant ostreid herpesvirus 1 (OsHV-1 µVar). Aquac Environ Interact 8:419–428
de Kantzow MC, Whittington RJ, Hick P (2019) Prior exposure to Ostreid herpesvirus 1 (OsHV-1) at 18 °C is associated with improved survival of juvenile Pacific oysters (Crassostrea gigas) following challenge at 22 °C. Aquaculture 507:443–450
Dégremont L (2011) Evidence of herpesvirus (OsHV-1) resistance in juvenile Crassostrea gigas selected for high resistance to the summer mortality phenomenon. Aquaculture 317:94–98
Dishon A, Davidovich M, Ilouze M, Kotler M (2007) Persistence of Cyprinid Herpesvirus 3 in infected cultured carp cells. J Virol 81:4828–4836
EFSA (2010) European Food Safety Authority Scientific Opinion of the Panel on Animal Health and Welfare on a request from the European Commission on the increased mortality events in Pacific oysters Crassostrea gigas. EFSA 8:1894–1953
EFSA (2015) European Food Safety Authority Panel on Animal Health Welfare. Oyster mortality. Eur Food Saf Auth J 13
Eide KE, Miller-Morgan T, Heidel JR, Kent ML, Bildfell RJ, Lapatra S, Watson G, Jin L (2011) Investigation of koi herpesvirus latency in koi. J Virol 85:4954–4962
Evans O, Paul-Pont I, Hick P, Whittington RJ (2014) A simple centrifugation method for improving the detection of Ostreid herpesvirus-1 (OsHV-1) in natural seawater samples with an assessment of the potential for particulate attachment. J Virol Methods 210:59–66
Evans O, Hick P, Whittington RJ (2017) Detection of Ostreid herpesvirus-1 microvariants in healthy Crassostrea gigas following disease events and their possible role as reservoirs of infection. J Invertebr Pathol 148:20–33
Green TJ, Montagnani C, Benkendorff K, Robinson N, Speck P (2014) Ontogeny and water temperature influences the antiviral response of the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol 36:151–157
Hick P, Evans O, Looi R, English C, Whittington RJ (2016) Stability of Ostreid herpesvirus-1 (OsHV-1) and assessment of disinfection of seawater and oyster tissues using a bioassay. Aquaculture 450:412–421
Hick PM, Evans O, Rubio A, Dhand NK, Whittington RJ (2018) Both age and size influence susceptibility of Pacific oysters (Crassostrea gigas) to disease caused by Ostreid herpesvirus-1 (OsHV-1) in replicated field and laboratory experiments. Aquaculture 489:110–120
Jenkins C, Hick P, Gabor M, Spiers Z, Fell SA, Gu X, Read A, Go J, Dove M, O’Connor W, Kirkland PD, Frances J (2013) Identification and characterisation of an Ostreid herpesvirus-1 microvariant (OsHV-1 mu-var) in Crassostrea gigas (Pacific oysters) in Australia. Dis Aquat Org 105:109–126
Keeling SE, Brosnahan CL, Williams R, Gias E, Hannah M, Bueno R, McDonald WL, Johnston C (2014) New Zealand juvenile oyster mortality associated with ostreid herpesvirus 1-an opportunistic longitudinal study. Dis Aquat Org 109:231–239
Klein J (1989) Are invertebrates capable of anticipatory immune responses? Scand J Immunol 29:499–505
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 1(13)
Martenot C, Oden E, Travaille E, Malas JP, Houssin M (2010) Comparison of two real-time PCR methods for detection of ostreid herpesvirus 1 in the Pacific oyster Crassostrea gigas. J Virol Methods 170:86–89
Mletzko A, Amtmann A, Bergmann S, Lee P, Christian J, Buchholz R, Becker A (2017) Inoculation of Cyprinid herpesvirus 3 (CyHV-3) on common carp brain cells—influence of process parameters on virus yield. In Vitro Cell Dev Biol Anim 53:579–585
Normand J, Blin JL, Jouaux A (2014) Rearing practices identified as risk factors for Ostreid herpesvirus 1 (OsHV-1) infection in Pacific oyster Crassostrea gigas spat. Dis Aquat Org 110:201–211
Oden E, Martenot C, Berthaux M, Travaille E, Malas JP, Houssin M (2011) Quantification of ostreid herpesvirus 1 (OsHV-1) in Crassostrea gigas by real-time PCR: determination of a viral load threshold to prevent summer mortalities. Aquaculture 317:27–31
Paul-Pont I, Dhand NK, Whittington RJ (2013) Spatial distribution of mortality in Pacific oysters Crassostrea gigas: reflection on mechanisms of OsHV-1 transmission. Dis Aquat Org 105:127–138
Paul-Pont I, Dhand NK, Whittington RJ (2013) Influence of husbandry practices on OsHV-1 associated mortality of Pacific oysters Crassostrea gigas. Aquaculture 412:202–214
Paul-Pont I, Evans O, Dhand NK, Rubio A, Coad P, Whittington RJ (2014) Descriptive epidemiology of mass mortality due to Ostreid herpesvirus-1 (OsHV-1) in commercially farmed Pacific oysters (Crassostrea gigas) in the Hawkesbury River estuary, Australia. Aquaculture 422:146–159
Paul-Pont I, Evans O, Dhand NK, Whittington RJ (2015) Experimental infections of Pacific oyster Crassostrea gigas using the Australian ostreid herpesvirus-1 (OsHV-1) mu Var strain. Dis Aquat Org 113:137–147
Pernet F, Barret J, Le Gall P, Corporeau C, Degremont L, Lagarde F, Pepin JF, Keck N (2012) Mass mortalities of Pacific oysters Crassostrea gigas reflect infectious diseases and vary with farming practices in the Mediterranean Thau lagoon, France. Aquac Environ Interact 2:215–237
Pernet F, Tamayo D, Petton B (2015) Influence of low temperatures on the survival of the Pacific oyster (Crassostrea gigas) infected with ostreid herpes virus type 1. Aquaculture 445
Pernet F, Lupo C, Bacher C, Whittington RJ (2016) Infectious diseases in oyster aquaculture require a new integrated approach. Philos Trans R Soc B Biol Sci 371:371
Petton B, Pernet F, Robert R, Boudry P (2013) Temperature influence on pathogen transmission and subsequent mortalities in juvenile Pacific oysters Crassostrea gigas. Aquac Environ Interact 3:257–273
Petton B, Boudry P, Alunno-Bruscia M, Pernet F (2015) Factors influencing disease-induced mortality of Pacific oysters Crassostrea gigas. Aquac Environ Interact 6:205–222
Raadsma HW, Thomson PC, Zenger KR, Cavanagh C, Lam MK, Jonas E, Jones M, Attard G, Palmer D, Nicholas FW (2009) Mapping quantitative trait loci (QTL) in sheep. I. A new male framework linkage map and QTL for growth rate and body weight. Genet Select Evol 41:34
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org
Renault T, Bouquet AL, Maurice JT, Lupo C, Blachier P (2014) Ostreid herpesvirus 1 infection among Pacific oyster (Crassostrea gigas) spat: relevance of water temperature to virus replication and circulation prior to the onset of mortality. Appl Environ Microbiol 80:5419–5426
Schikorski D, Faury N, Pépin JF, Saulnier D, Tourbiez D, Renault T (2011) Experimental ostreid herpesvirus 1 infection of the Pacific oyster Crassostrea gigas: kinetics of virus DNA detection by q-PCR in seawater and in oyster samples. Virus Res 155:28–34
Schikorski D, Renault T, Saulnier D, Faury N, Moreau P, Pepin JF (2011) Experimental infection of Pacific oyster Crassostrea gigas spat by ostreid herpesvirus 1: demonstration of oyster spat susceptibility. Vet Res 42:27
Segarra A, Mauduit F, Faury N, Trancart S, Dégremont L, Tourbiez D, Haffner P, Barbosa-Solomieu V, Pépin J-F, Travers M-A, Renault T (2014) Dual transcriptomics of virus-host interactions: comparing two Pacific oyster families presenting contrasted susceptibility to ostreid herpesvirus 1. BMC Genomics 15:580
Segarra A, Baillon L, Faury N, Tourbiez D, Renault T (2016) Detection and distribution of Ostreid herpesvirus 1 in experimentally infected Pacific oyster spat. J Invertebr Pathol 133:59–65
Stevens JG (1994) Overview of herpesvirus latency. Semin Virol 5:191–196
Ugalde SC, Preston J, Ogier E, Crawford C (2018) Analysis of farm management strategies following herpesvirus (OsHV-1) disease outbreaks in Pacific oysters in Tasmania, Australia. Aquaculture 495:179–186
Weiliang Q, Chavarro J, Lazarus R, Rosner B, Ma J (2018) powerSurvEpi: power and sample size calculation for survival analysis of epidemiological studies. R package version 0.1.0
Whittington RJ, Dhand NK, Evans O, Paul-Pont I (2015) Further observations on the influence of husbandry practices on OsHV-1 mu Var mortality in Pacific oysters Crassostrea gigas: age, cultivation structures and growing height. Aquaculture 438:82–97
Whittington RJ, Hick PM, Evans O, Rubio A, Alford B, Dhand N, Paul-Pont I (2015) Protection of Pacific oyster (Crassostrea gigas) spat from mortality due to ostreid herpesvirus 1 (OsHV-1 μVar) using simple treatments of incoming seawater in land-based upwellers. Aquaculture 437:10–20
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York
Xu JR, Bently J, Beck L, Reed A, Miller-Morgan T, Heidel JR, Kent ML, Rockey DD, Jin L (2013) Analysis of koi herpesvirus latency in wild common carp and ornamental koi in Oregon, USA. J Virol Methods 187:372–373
Acknowledgements
This research was funded by the Fisheries Research and Development Corporation (Grant: FRDC 2014-040) and The University of Sydney. Oysters were donated by Shellfish Culture, Tasmania and grown by Bruce Alford. The authors would like to acknowledge Alison Tweedie, Slavicka Patten, Stuart Glover and Craig Kristo for technical assistance. Chris Howden of the Sydney Informatics Hub is thanked for advice on statistics. Ethics approval was not required under Australian and institutional animal ethics committee guidelines for this study. The authors declare they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Chan-Shing Lin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Kantzow, M.C., Whittington, R.J. & Hick, P.M. Different in vivo growth of ostreid herpesvirus 1 at 18 °C and 22 °C alters mortality of Pacific oysters (Crassostrea gigas). Arch Virol 164, 3035–3043 (2019). https://doi.org/10.1007/s00705-019-04427-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-019-04427-2