Abstract
We report a long-term treatment of Parkinson’s disease in out-patient clinics. The patients with Parkinson’s disease were evaluated at the time of clinic visit from September 1st, 2015 to February 29th, 2016. Total number of the patients was 498. The age at the evaluation was 69.9 ± 9.3 years and the age of onset was 60.2 ± 11.3. Hoehn and Yahr severity was 3.28 ± 0.94 in patients who were from 16 to 20 years (n = 53) and 3.00 ± 0.86 in patients from 21 years or more (n = 38) from the onset of the disease to the evaluation. The dose of levodopa was 741 ± 295 mg per day and the number of levodopa dosing was 5.85 ± 2.59 times in 16–20 years from the onset to the evaluation and 703 ± 251 mg/day and 6.03 ± 3.20 times a day in 21 years or more from the onset to the evaluation. Levodopa was given in most cases into an empty stomach. The incidence of wearing off was 73.6% and dyskinesia was 37.7% in the 16–20 years group and 76.3% and 55.3% in 21 years or more group, respectively. The patients who had 15 years or less from the onset to the evaluation had much milder severity of the disease. Hoehn and Yahr severity, the dose of levodopa, and the incidence of wearing off were about the same as in the literature. But the incidence of dyskinesia was much lower than those appeared in the literature. We discussed reasons why the incidence of dyskinesia was lower in our study.
Similar content being viewed by others
Introduction
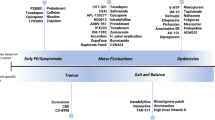
It is not well known about the ideal long-term treatment of Parkinson’s disease (PD). Initial treatment with levodopa is in most of the patients smooth. At the first motor symptom of PD, still approximately 50% of 18F-dopa uptake in the striatum is remaining (Brooks 2004). Therefore, levodopa is likely taken up into the remaining dopaminergic neurons, which are equipped with the dopamine transporters. Therefore, there will be no wearing off or dyskinesia. Dopamine once released to the synaptic cleft is rapidly taken up by the dopamine transporter to the original dopaminergic neurons. Thus, dopamine is re-used again when it is necessary.
Then the degeneration of dopaminergic neurons in the substantia nigra progresses slowly in PD despite the treatment with levodopa. Then the patients with PD realize that some worsening of their symptoms before the next dose of levodopa. Then the physicians in charge have to prescribe levodopa four times or more a day. This is the wearing off. In addition, physicians in charge may have to prescribe levodopa when the patients’ stomachs are empty to get a maximum effect of levodopa.
How about the frequencies of wearing off and dyskinesia in the literature? Wearing off fluctuations have been reported to be 40–50% of patients treated with levodopa for 5 years and approximately two-thirds in patients treated for 10 years or more. Dyskinesias have been reported to be 40–50% in patients treated with levodopa for 5–10 years or more (Caraceni et al. 1991; Reardon et al. 1999; Schrag and Quinn 2000; Rascol et al. 2000: Ahlskog and Muenter 2001; Parkinson Study Group 2004; Hauser et al. 2007; Parkinson Study Group CALM Cohort Investigators 2009: Colombo et al. 2015). The longer the treatment period, the more patients would show dyskinesia.
At this stage of levodopa treatment when the wearing off is present, where levodopa would be decarboxylated to dopamine? Levodopa is taken up by the aromatic amino acid uptake system to the cells, i.e., dopamine, noradrenalin, and serotonin neurons but also non-neuronal tissues. Levodopa is still effective although the duration of on period is shortened. In addition, no dopamine transporter can be visualized in the DAT scan in the posterior putamen where the motor loop to the frontal cortex is of upmost importance in voluntary movements. Here levodopa would be decarboxylated to dopamine at least in part in the serotonergic neurons coming from the raphe nucleus in the pons. The serotonergic neurons are equipped with the serotonin transporter but not the dopamine transporter. Therefore, dopamine released from the serotonergic neurons is not taken up through the serotonin transporter. Thus, dopamine released into the synaptic clef will be metabolized by monoamine oxidase and catechol-O-methyltransferase. Therefore, dopamine in the serotonergic neurons would not stay long in the basal ganglia. We believe that this is one of the cellular mechanisms for wearing off. When the synaptic cleft dopamine is too much, dyskinesia may result. In recent years, a number of articles suggested the serotonergic neurons for decarboxylation of dopamine (Bara-Jimenez et al. 2005; Cheshire and Williams 2012; Politis et al. 2014; Smith et al. 2015; Cheshire et al. 2015; Lee et al. 2015; Roussakis et al. 2016).
Keep these facts in mind, we treated patients with PD so that the long-term complications of levodopa will become as low as possible.
Methods
Style
This is an observational study. The patients with PD were evaluated at the time of clinic visits from September 1st, 2015 to February 29th, 2016.
Diagnosis of PD
Those patients who fulfilled the following criteria were included, i.e., presence of parkinsonism according to the British Council definition (Hughes et al. 1992), reduced uptake of cardiac meta-iodobenzylguanidine (MIBG), and normal brain MRI other than the aging-related changes. In some patients, cardiac MIBG was not examined; in such cases, response to levodopa was confirmed.
Drug treatment
Levodopa was used when the patients were 65 years or older. When the patients were less than 60 years, a dopamine agonist was used. When the patients were 60 years or older but not reaching the 65 years, a case by case principle was adopted.
Dose of levodopa
As an initial dose, we gave to the patients 200–300 mg of levodopa with a decarboxylase inhibitor (DCI) in a day in two to three divided doses after each meal. When this amount did not produce a satisfactory result, levodopa with DCI was given shortly before the meal. Still the amount of levodopa appeared to be not enough, the levodopa dose was increased gradually to 450 mg or to 600 mg a day. If this amount still is not satisfactory to improve patients’ parkinsonian symptoms, other drugs were added (see below). When the patient had wearing off phenomenon, levodopa with DCI was given according to the length of the on period. When the effect of levodopa wears off, we instructed the patients to take the next dose of levodopa right after the symptoms of wearing off. Each dose of levodopa was 50–200 mg in this case. If a patient had non-motor symptoms such as headache, lumbago, or any other symptoms that lead to off state, levodopa with DCI was given at the onset of such non-motor symptoms.
When a patient developed inter-dose dyskinesia, each levodopa dose was decreased when it was possible, and the number of giving levodopa in a day was increased so that the daily dose of levodopa would not change much. Other anti-parkinsonian drugs was reduced or discontinued if possible except for the anti-cholinergics and amantadine HCl. Amantadine HCL was prescribed at times to decrease dyskinesia.
Other medication
Other medications such as a monoamine oxidase B inhibitor (selegiline), catechol-O-methyltransferase inhibitor (entacapone), dopamine agonists (pramipexole, ropinirole, rotigotine, or pergolide), trihexyphenidyl, amantadine HCl, zonisamide, istradefylline, and/or dops were used when indicated. Entacapone (100 mg at each time) was used at the same time with levodopa. When levodopa was given nine times or more in a day, the last dose was given without entacapone.
Treatment of non-motor symptoms
Constipation, nocturia, hypotension, pre-tibial pitting edema, pain, sleep disorders, excessive daytime sleepiness, anxiety state, depression, fatigue, impulse control disorders, dopamine dysregulation syndromes, hallucinations, delusions, psychosis, and dementia were treated as possible with appropriate drugs.
Semi-quantitative evaluation
Hoehn and Yahr severity of the disease was evaluated when the patients were on. Resting tremor, rigidity, and bradykinesia were semi-quantitatively evaluated according to the UPDRS. Gait disturbance was evaluated according to the following criteria; 0, normal; 1, mild gait disturbance; 2, moderated gait disturbance; the patient would not fall when he or she walks alone, 3, marked gait disturbance; the patient would fall when he or she walks alone, 4, unable to walk. Retropulsion was evaluated according to the following criteria: 0, no retropulsion; 1, mild retropulsion; the patient would recover and he or she would not fall; 2, moderate retropulsion, the patient would fall when he or she is not supported; 3, marked retropulsion, the patient would fall when he or she stands alone; 4, unable to stand because of unsteadiness of standing. Wearing off was evaluated according to the following criteria; 0, no wearing off; 1, mild wearing off; taking levodopa four time or less in a day because of wearing off, 2, moderated wearing off; taking levodopa 5–7 times in a day, 3, marked wearing off; taking levodopa 8–10 times in a day, 4, severe wearing off; taking levodopa 11 times or more in a day. Dyskinesia was evaluated according to the following criteria; 0, no dyskinesia; 1, mild dyskinesia; the patient may not realize the presence of dyskinesia, 2, moderate dyskinesia, the patient realizes the dyskinesia; but not bothered by the dyskinesia in the daily life, 3, marked dyskinesia, the patient realizes the dyskinesia and bothered by the dyskinesia in the daily life; 4, severe dyskinesia, the patient feels fatigue and/or difficulty in the daily life because of dyskinesia. Freezing was evaluated according to the following criteria: 0, no freezing; 1, mild freezing; the freezing occurs at home or outside of the home, but not in the examining room; 2, moderate freezing; the freezing occurs at home and/or outside of the home, and in the examining room. Hallucination was evaluated according to the following criteria; 0, none; 1, mild hallucination; the patient realizes that it is the hallucination but not bothered from it, 2, moderate hallucination; hallucinations occur mainly during night, patients realize that it is hallucination and sometime embarrassing and annoying. 3, marked hallucination; hallucinations occur day and night, patients may not realize that it is the hallucination. 4, severe hallucination; the patient does not realize that it is the hallucination and the patient may talk to the hallucination; the patient may lose orientation or may be excited. Dementia was evaluated according to the following criteria, 0, no dementia; 1, mild dementia; dementia does not need observation and it does not interfere with the daily life; 2, moderate dementia; dementia may need observation, 3, marked dementia; dementia needs observation, but he or she is oriented to the outline of the home and its vicinity; 4, severe dementia, dementia needs observation; he or she is disoriented to the home and its vicinity.
Statistical analysis
Values represent mean and standard deviation where Hoehn and Yahr severity was transformed into integer score. Mean age at onset was compared between male and female by Student’s t test. Average score of Hoehn and Yahr severity was compared by Wilcoxon rank-sum test.
Results
Numbers of patients
The total number of the patients was 498 (male 222, female 276).
The age at evaluation and the age of onset
The time intervals from the onset of the disease and the time of the evaluation were divided into 5 groups, i.e., the interval between the onset and the evaluation 5 years or less, 6–10 years, 11–15 years, 16–20 years, and 21 years or more (Table 1). The age at the evaluation was 69–71 in average in all groups. The ages at the onset were gradually decreased as the observation period increased.
There was no significant difference between the male and the female in the age and the age of onset. The age at the evaluation was 70.0 ± 9.5 years in males, 69.9 ± 9.1 years in females (ns), and 69.9 ± 9.3 years in total. The age of onset was 58.9 ± 12.3 years in males, 60.5 ± 10.5 years in females (non-significant), and 60.2 ± 11.3 years in total.
Symptoms at the age of onset
Symptoms at the onset were tremor in 49.8%, gait disturbance in 33.3%, and symptoms related to bradykinesia in 16.9%. Bilateral involvement with tremor at the onset was rare. In the remaining, those patients who had gait disturbance at the onset had unilateral or bilateral involvement, and those who had symptoms related to bradykinesia had usually unilateral hand involvement.
Hoehn and Yahr severity
Hoehn and Yahr severity of the patients who had the tremor at the onset (2.52 ± 1.01) was less than the patients who had gait disturbance or bradykinesia at the onset (2.80 ± 0.96) (p < 0.0001). There was no significant difference in the age and the age of onset between these two groups. Hoehn and Yahr severity according to the breakdown of the intervals between the age of onset and the age of the evaluation is shown in Table 2. Hoehn and Yahr severity increased from the group of 5 years or less to the group of 16–20 years from the onset to the evaluation (3.28 ± 0.94). But it was less than that in the group of 21 years or more (3.00 ± 0.86). This was in part due to the presence of early onset PD with good prognoses in the group of 21 years or more.
Dose of levodopa
Doses of levodopa are shown in Table 3. It increased from the group of 5 years or less from the onset to the group of 16–20 years from the onset (416 ± 223 to 741 ± 295 mg a day). It was somewhat less in the group of 21 years or more from the onset (703 ± 251 mg). Numbers of levodopa doses increased from the group of 5 years or less to the group of 21 years or more (3.29 ± 1.52 to 6.03 ± 3.20).
Other anti-PD drugs
Other anti-PD drugs, which were taken by the patients at the time of the evaluations, are listed in Table 4. Dopamine agonists and trihexyphenidyl were the two most concomitant drugs with levodopa. They were used in approximately half of the patients.
Tremor, rigidity, bradykinesia, gait disturbance, and retropulsion
The numbers of patients who had these symptoms at the time of the evaluation are listed in Table 5. At the time of onset, tremor was present in about 50% of the patients. But it reduced to 23.0% to the group of 5 years or less and further reduced in the remaining groups to approximately 15%. The overall presence of tremor was 16.1%.
Rigidity remained in 41.6% of the patients, but in many of the patients, it was usually present in the neck and usually milder or none in the extremities. Bradykinesia remained in 84.5% and gait disturbance in 80.3% of the patients. These were the two major symptoms, which remained in most of the patients. Retropulsion was remaining in 33.3% of the patients. In many cases, retropulsion was present initially but it tended to disappear in subsequent years.
Wearing off, dyskinesia, freezing, hallucination, and dementia
Wearing off was only 17.8% in the group of 5 years or less, but it increased to 56.3% in the group of 11–15 years; it reached approximately 75% thereafter (Table 6). Dyskinesia was noted only in 2.2% in the group of 5 years or less from the onset. It was still 17.7% in the group of 6–10 years. In the group of 11–15 years, it increased to 31.6%, and in the group of 16–20 years, to 37.7%. In the group of 21 years or more, it was 55.3%, apparently in part due to the increase of early onset patients. Freezing increased from 30.4% in the group of 5 years or less to 76.3% in the group of 21 years or more. Hallucinations (mainly visual) were not often (5.6%). Apparently, this was mainly due to the treatment effects: those patients who had hallucinations were treated by donepezil or quetiapine or both, and in many patients hallucinations disappeared at the time of the evaluation. Dementia was noted in 20.5% as the total. It was highest in the group of 16–20 years from the onset to the evaluation (39.6%).
Hoehn and Yahr severity, bradykinesia, and gait disturbance according to the age of onset
These are listed in Table 7. Patients with the age of onset 70 years or older had shortest period from the onset of disease to time of the evaluation. Despite that, these patients marked the worst value of the Hoehn and Yahr at the time of the evaluation. Bradykinesia and gait disturbance scores were the worst two in this group.
Discussion
By reviewing the above results, (1) the overall outcome in the Hoehn and Yahr severity (2.66 ± 0.99, disease duration 10.28 ± 7.05 years) is about the same as reported in the literature (Schrag and Quinn 2000, 2.4 ± 0.8, disease duration 6.8 ± 4.3 years; Nissinen et al. 2009, 2.4 ± 0.6, disease duration 10.9 ± 5.4 years). (2) Levodopa doses (613 ± 297 mg) and the numbers of drug intake in a day are approximately the same as in the literature (Rascol et al. 2000, 753 ± 398 mg; Parkinson Study Group 2004, 702 ± 461 mg; Nissinen et al. 2009, 692 ± 364 mg). (3) Use of trihexyphenidyl is more frequent (45%) than the recent literature but the dose of trihexyphenidyl is much smaller than that in the literature (Parkinson Study Group 2004, 4.7%; Nissinen et al. 2009, 16%). (4) The incidence of wearing off (17.8–76.3%, total mean 53.6% in 10.28 ± 7.05 years) is about the same as in the literature (Caraceni et al. 1991, 48% in 2–10 years; Reardon et al. 1999, 34% in 15 months; Schrag and Quinn 2000, 63% in 10 years or more; Parkinson Study Group 2004, 62.7% in 4 years; Hauser et al. 2007, 72.0% in 10 years; Parkinson Study Group CALM Cohort Investigators 2009, 58.8% in 6 years: Colombo et al. 2015, 63.7% for men in more than 5 years, 73.5% for women in more than 5 years of treatment with levodopa). (5) The frequency of dyskinesia, however, is much less (2.2–55.3%, total mean 21.7% in 10.28 ± 7.05 years) than those in the literature (Reardon et al. 1999, 53% in 15 months; Schrag and Quinn 2000, 53% in 10 years or more; Rascol et al. 2000, 45% in 5 years; Ahlskog and Muenter 2001, 40% in 4–6 years; Parkinson Study Group 2004, 54.0% in 4 years; Hauser et al. 2007, 77.8% in 10 years; Parkinson Study Group CALM Cohort Investigators 2009, 36.8% in 6 years of treatment with levodopa). One report from China reported low incidence of dyskinesia (Chen et al. 2014, 19.3% in 10 years or more of treatment with levodopa).
Frequency of dyskinesia was much lower than those in the literature, which we did not expect when the evaluations were done. In the group of patients who had PD for 16–20 years, the frequency of dyskinesia was 37.7%. As our study is an open study, we did various things when patients started to have dyskinesia such as frequent dosing of levodopa, lower amount of levodopa at each dose, reducing or discontinuing concomitant anti-Parkinson drugs such as a monoamine oxidase B inhibitor, entacapone, dopamine agonists, zonisamide, or istradefylline. We did not reduce amantadine HCl or trihexyphenidyl when used unless patients had hallucinations or cognitive or psychiatric side effects.
Today, the serotonergic neurons in the basal ganglia is claimed to be responsible for levodopa-induced dyskinesia. The earliest literature discussing the relationship between levodopa-induced dyskinesia and the serotonergic system in the striatum is that of Bara-Jimenez et al. (2005). They gave sarizotan, a serotonergic 5-HT1A agonist, to patients with advanced PD with dyskinesias at 2 and 5 mg twice a day to 18 PD patients with dyskinesia. They found that sarizotan co-administration reduced levodopa-induced dyskinesias. They suggested that 5-HT1A agonists might be useful as a levodopa adjuvant in the treatment of PD. Sprouting of the serotonergic neurons to the striatum in MPTP-lesioned mice is well known (Rozas et al. 1998; Lee et al. 2008). In experimental animals, dopa-decarboxylase-immuno-reactive neurons were noted in dopamine-depleted striata (Lopez-Real et al. 2003). Therefore, it seems likely that decarboxylation of levodopa takes place in serotonergic neurons in the striatum in advanced PD (Lopez et al. 2001; Bara-Jimenez et al. 2005; Yamada et al. 2007; Cheshire and Williams 2012; Politis et al. 2014; Smith et al. 2015; Cheshire et al. 2015; Lee et al. 2015). Lopez et al. (2001) observed that extrinsic levodopa given to dopamine denervated rats was still able to induce the rotational behavior and concluded that the serotonergic neurons in the striatum might be responsible for conversion of the exogenous levodopa to dopamine. Yamada et al. (2007) showed extrinsically levodopa-derived dopamine in the serotonergic neurons in the basal ganglia in rats. But these serotonergic neurons do not have dopamine transporters. The serotonin transporters cannot reuptake dopamine once released from the serotonergic neurons into the synaptic clefts (Mortensen et al. 1999; Mantovani et al. 2009). This inability to re-use dopamine would shorten the effect of levodopa, and the amount of dopamine released into synaptic cleft is too much, it would result in peak-dose dyskinesia.
Treatments with the serotonin agonist such as 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH-DPAT) were reported to reduce dyskinesia in experimental animals (Tomiyama et al. 2005; Carta et al. 2007; Muñoz et al. 2009; Nahimi et al. 2012; Iderberg et al. 2015; Paolone et al. 2015; McCreary et al. 2016; Ghiglieri et al. 2016). The serotonin agonists would reduce the activity of the serotonin neurons and thus it would decrease the release of serotonin as well as dopamine. Iderberg et al. (2015) and McCreary et al. (2016) proposed NLX-112 as a new potent serotonin 5-HT1A receptor agonist in experimental rats, and Paolone et al. (2015) and Ghiglieri et al. (2016) proposed eltoprazine as a new 5-HT1A/5-HT1B receptor agonist.
The serotonergic neurons undergo eventually neurodegeneration (Kienzl et al. 1981), but degree of neurodegeneration is much less than the dopaminergic neurons in the basal ganglia in PD (Kish et al. 2008; Buddhala et al. 2015). In addition to these data, studies on PD patients have been reported using tomography. Smith et al. (2015) studied the pallidal serotonergic neurons using a positron emission tomography in PD with dyskinesia. They used a (11)C-DASB positron emission tomography (PET), a marker of the serotonin transporter availability, and (11) a C-raclopride PET. They studied 12 PD patients with dyskinesia and 12 without dyskinesia. Levels of the GP (globus pallidus) serotonin transporter binding correlated positively with the severity of dyskinesias and they concluded that the higher GP serotonergic function was associated with levodopa-induced dyskinesias in PD. Recently, Lee et al. (2015) studied 30 patients with PD without depression or dementia, and they classified their patients into three groups, one was the non-dyskinetic, one was the dyskinetic, and the other drug naïve. They noted highest binding potential ratios ((11)C-DASB/(18)F-FP-CIT) at the putamen, which indicated serotoninergic fiber innervation relative to dopaminergic fiber availability. The binding ratio was highest in the dyskinetic group, followed by the non-dyskinetic and the drug-naive PD groups. They suggested that the serotonin/dopamine transporter ratio might be a potential marker of disease progression and an indicator of risk for levodopa-induced dyskinesia in PD. Roussakis et al. (2016) made essentially the similar observations recently. Cheshire et al. (2015) obtained the brain tissue from 44 PD patients and 17 age-matched controls and measured the dopaminergic and serotonergic markers. They found marked loss of the dopaminergic transporters but not in the serotonergic transporters in the posterior putamen of the PD patients. But the serotonergic receptor densities were not different from the dyskinetic and the non-dyskinetic patients and they concluded that the serotonergic system was not a risk factor for developing dyskinesias in PD.
Although striatal cholinergic system may influence the levodopa-induced dyskinesia, still the results of animal experiments were present (Bordia et al. 2016), and a nicotine receptor α7 agonist AQW051 had no effect on the levodopa-induced dyskinesia in patients with PD (Trenkwalder et al. 2016).
The reason why the frequency of dyskinesia was lower than those in the western countries is not well known because this is an observational study. But whenever dyskinesias appeared in the patients in our study, the individual doses of levodopa were reduced, if possible, and the numbers of drug intakes were increased, so that the total daily doses of levodopa were approximately the same as those before, and the numbers of drugs other than levodopa were reduced as possible. We believe that these modifications of the anti-parkinsonian drugs might result in the lower frequency of dyskinesia in our study.
References
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16:448–458
Bara-Jimenez W, Bibbiani F, Morris MJ, Dimitrova T, Sherzai A, Mouradian MM, Chase TN (2005) Effects of serotonin 5-HT1A agonist in advanced Parkinson’s disease. Mov Disord 20:932–936
Bordia T, Perez XA, Heiss JE, Zhang D, Quik M (2016) Optogenetic activation of striatal cholinergic interneurons regulates l-dopa-induced dyskinesias. Neurobiol Dis 91:47–58. doi:10.1016/j.nbd.2016.02.019
Brooks DJ (2004) Neuroimaging in Parkinson’s disease. NeuroRx 1:243–254
Buddhala C, Loftin SK, Kuley BM, Cairns NJ, Campbell MC, Perlmutter JS, Kotzbauer PT (2015) Dopaminergic, serotonergic, and noradrenergic deficits in Parkinson disease. Ann Clin Transl Neurol 2:949–959. doi:10.1002/acn3.246
Caraceni T, Scigliano G, Musicco M (1991) The occurrence of motor fluctuations in parkinsonian patients treated long term with levodopa: role of early treatment and disease progression. Neurology 41:380–384
Carta M, Carlsson T, Kirik D, Björklund A (2007) Dopamine released from 5-HT terminals is the cause of l-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833
Chen W, Xiao Q, Shao M et al (2014) Prevalence of wearing-off and dyskinesia among the patients with Parkinson’s disease on levodopa therapy: a multi-center registry survey in mainland China. Transl Neurodegener 3:26. doi:10.1186/2047-9158-3-26
Cheshire PA, Williams DR (2012) Serotonergic involvement in levodopa-induced dyskinesias in Parkinson’s disease. J Clin Neurosci 19:343–348. doi:10.1016/j.jocn.2011.09.008
Cheshire P, Ayton S, Bertram KL et al (2015) Serotonergic markers in Parkinson’s disease and levodopa-induced dyskinesias. Mov Disord 30:796–804. doi:10.1002/mds.26144
Colombo D, Abbruzzese G, Antonini A et al (2015) The “gender factor” in wearing-off among patients with Parkinson’s disease: a post hoc analysis of DEEP study. Sci World J. 2015:787451. doi:10.1155/2015/787451
Ghiglieri V, Mineo D, Vannelli A et al (2016) Modulation of serotonergic transmission by eltoprazine in L-DOPA-induced dyskinesia: behavioral, molecular, and synaptic mechanisms. Neurobiol Dis 86:140–153. doi:10.1016/j.nbd.2015.11.022
Hauser RA, Rascol O, Korczyn AD et al (2007) Ten-year follow-up of Parkinson’s disease patients randomized to initial therapy with ropinirole or levodopa. Mov Disord 22:2409–2417
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Iderberg H, McCreary AC, Varney MA (2015) NLX-112, a novel 5-HT1A receptor agonist for the treatment of L-DOPA-induced dyskinesia: behavioral and neurochemical profile in rat. Exp Neurol 271:335–350. doi:10.1016/j.expneurol.2015.05.021
Kienzl E, Riederer P, Jellinger K, Wesemann W (1981) Transitional states of central serotonin receptors in Parkinson’s disease. J Neural Transm 51:113–122
Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang LJ, Guttman M, Furukawa Y (2008) Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain 131:120–131
Lee J, Zhu WM, Stanic D (2008) Sprouting of dopamine terminals and altered dopamine release and uptake in Parkinsonian dyskinaesia. Brain 131:1574–1587. doi:10.1093/brain/awn085
Lee JY, Seo S, Lee JS, Kim HJ, Kim YK, Jeon BS (2015) Putaminal serotonergic innervation: monitoring dyskinesia risk in Parkinson disease. Neurology 85:853–860. doi:10.1212/WNL.0000000000001909
Lopez A, Muñoz A, Guerra MJ, Labandeira-Garcia JL (2001) Mechanisms of the effects of exogenous levodopa on the dopamine-denervated striatum. Neuroscience 103:639–651
Lopez-Real A, Rodriguez-Pallares J, Guerra MJ, Labandeira-Garcia JL (2003) Localization and functional significance of striatal neurons immunoreactive to aromatic l-amino acid decarboxylase or tyrosine hydroxylase in rat Parkinsonian models. Brain Res 969:135–146
Mantovani M, Dooley DJ, Weyerbrock A, Jackisch R, Feuerstein TJ (2009) Differential inhibitory effects of drugs acting at the noradrenaline and 5-hydroxytryptamine transporters in rat and human neocortical synaptosomes. Br J Pharmacol 158:1848–1856. doi:10.1111/j.1476-5381.2009.00478.x
McCreary AC, Varney MA, Newman-Tancredi A (2016) The novel 5-HT1A receptor agonist, NLX-112 reduces l-DOPA-induced abnormal involuntary movements in rat: a chronic administration study with microdialysis measurements. Neuropharmacology 105:651–660. doi:10.1016/j.neuropharm.2016.01.013
Mortensen OV, Kristensen AS, Rudnick G, Wiborg O (1999) Molecular cloning, expression and characterization of a bovine serotonin transporter. Brain Res Mol Brain Res 71:120–126
Muñoz A, Carlsson T, Tronci E, Kirik D, Björklund A, Carta M (2009) Serotonin neuron-dependent and -independent reduction of dyskinesia by 5-HT1A and 5-HT1B receptor agonists in the rat Parkinson model. Exp Neurol 219:298–307. doi:10.1016/j.expneurol.2009.05.033
Nahimi A, Høltzermann M, Landau AM et al (2012) Serotonergic modulation of receptor occupancy in rats treated with l-DOPA after unilateral 6-OHDA lesioning. J Neurochem 120:806–817. doi:10.1111/j.1471-4159.2011.07598.x
Nissinen H, Kuoppamäki M, Leinonen M, Schapira AH (2009) Early versus delayed initiation of entacapone in levodopa-treated patients with Parkinson’s disease: a long-term, retrospective analysis. Eur J Neurol 16:1305–1311. doi:10.1111/j.1468-1331.2009.02726.x
Paolone G, Brugnoli A, Arcuri L, Mercatelli D, Morari M (2015) Eltoprazine prevents levodopa-induced dyskinesias by reducing striatal glutamate and direct pathway activity. Mov Disord 30:1728–1738. doi:10.1002/mds.26326
Parkinson Study Group (2004) Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 61:1044–1053
Parkinson Study Group CALM Cohort Investigators (2009) Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease. Arch Neurol 66:563–570. doi:10.1001/archneur.66.1.nct90001
Politis M, Wu K, Loane C et al (2014) Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson’s disease patients. J Clin Invest 124:1340–1349. doi:10.1172/JCI71640
Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med 342:1484–1491
Reardon KA, Shiff M, Kempster PA (1999) Evolution of motor fluctuations in Parkinson’s disease: a longitudinal study over 6 years. Mov Disord 14:605–611
Roussakis AA, Politis M, Towey D, Piccini P (2016) Serotonin-to-dopamine transporter ratios in Parkinson disease: relevance for dyskinesias. Neurology 86:1152–1158. doi:10.1212/WNL.0000000000002494
Rozas G, Liste I, Guerra MJ, Labandeira-Garcia JL (1998) Sprouting of the serotonergic afferents into striatum after selective lesion of the dopaminergic system by MPTP in adult mice. Neurosci Lett 245:151–154
Schrag A, Quinn N (2000) Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain 123:2297–2305
Smith R, Wu K, Hart T et al (2015) The role of pallidal serotonergic function in Parkinson’s disease dyskinesias: a positron emission tomography study. Neurobiol Aging 36:1736–1742. doi:10.1016/j.neurobiolaging.2014.12.037
Tomiyama M, Kimura T, Maeda T, Kannari K, Matsunaga M, Baba M (2005) A serotonin 5-HT1A receptor agonist prevents behavioral sensitization to L-DOPA in a rodent model of Parkinson’s disease. Neurosci Res 52:185–194
Trenkwalder C, Berg D, Rascol O et al (2016) A placebo-controlled trial of AQW051 in patients with moderate to severe levodopa-induced dyskinesia. Mov Disord 31:1049–1054. doi:10.1002/mds.26569
Yamada H, Aimi Y, Nagatsu I, Taki K, Kudo M, Arai R (2007) Immunohistochemical detection of l-DOPA-derived dopamine within serotonergic fibers in the striatum and the substantia nigra pars reticulata in Parkinsonian model rats. Neurosci Res 59:1–7
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mizuno, Y., Shimoda, S. & Origasa, H. Long-term treatment of Parkinson’s disease with levodopa and other adjunctive drugs. J Neural Transm 125, 35–43 (2018). https://doi.org/10.1007/s00702-016-1671-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1671-x