Abstract
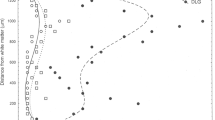
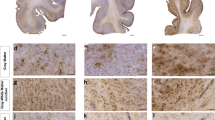
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disorder which may result from repetitive brain injury. A variety of tau-immunoreactive pathologies are present, including neurofibrillary tangles (NFT), neuropil threads (NT), dot-like grains (DLG), astrocytic tangles (AT), and occasional neuritic plaques (NP). In tauopathies, cellular inclusions in the cortex are clustered within specific laminae, the clusters being regularly distributed parallel to the pia mater. To determine whether a similar spatial pattern is present in CTE, clustering of the tau-immunoreactive pathology was studied in the cortex, hippocampus, and dentate gyrus in 11 cases of CTE and 7 cases of Alzheimer’s disease neuropathologic change (ADNC) without CTE. In CTE: (1) all aspects of tau-immunoreactive pathology were clustered and the clusters were frequently regularly distributed parallel to the tissue boundary, (2) clustering was similar in two CTE cases with minimal co-pathology compared with cases with associated ADNC or TDP-43 proteinopathy, (3) in a proportion of cortical gyri, estimated cluster size was similar to that of cell columns of the cortico-cortical pathways, and (4) clusters of the tau-immunoreactive pathology were infrequently spatially correlated with blood vessels. The NFT and NP in ADNC without CTE were less frequently randomly or uniformly distributed and more frequently in defined clusters than in CTE. Hence, the spatial pattern of the tau-immunoreactive pathology observed in CTE is typical of the tauopathies but with some distinct differences compared to ADNC alone. The spread of pathogenic tau along anatomical pathways could be a factor in the pathogenesis of the disease.


Similar content being viewed by others
References
Armstrong RA (1993a) Is the clustering of neurofibrillary tangles in Alzheimer’s patients related to the cells of origin of specific cortico-cortical projections? Neurosci Lett 160:57–60
Armstrong RA (1993b) The usefulness of spatial pattern analysis in understanding the pathogenesis of neurodegenerative disorders, with particular reference to plaque formation in Alzheimer’s disease. Neurodegeneration 2:73–80
Armstrong RA (1997) Analysis of spatial patterns in histological sections of brain tissue. J Neurosci Methods 73:141–147
Armstrong RA (2003a) Quantifying the pathology of neurodegenerative disorders: quantitative measurements, sampling strategies and data analysis. Histopathology 42:521–529
Armstrong RA (2003b) Measuring the degree of spatial correlation between histological features in thin sections of brain tissue. Neuropathology 23:245–253
Armstrong RA (2006a) Classic β-amyloid deposits cluster around large diameter blood vessels rather than capillaries in sporadic Alzheimer’s disease. Curr Neurovasc Res 3:289–294
Armstrong RA (2006b) Measuring the spatial arrangement patterns of pathological lesions in histological sections of brain tissue. J Microsc 221:153–158
Armstrong RA (2009) Spatial correlations between the vacuolation, prion protein (PrPsc) deposits and cerebral blood vessels in sporadic Creutzfeldt–Jacob disease. Curr Neurovasc Res 6:239–245
Armstrong RA, Cairns NJ (2009) Clustering and spatial correlations of the neuronal cytoplasmic inclusions, astrocytic plaques and ballooned neurons in corticobasal degeneration. J Neural Transm 116:1103–1110
Armstrong RA, Cairns NJ (2012) Different molecular pathologies result in similar spatial patterns of cellular inclusions in neurodegenerative disease: a comparative study of eight disorders. J Neural Transm 119:1551–1560
Armstrong RA, Cairns NJ, Lantos PL (1998a) Clustering of Pick bodies in Pick’s disease. Neurosci Lett 242:81–84
Armstrong RA, Cairns NJ, Lantos PL (1998b) Spatial distribution of diffuse, primitive, and classic amyloid-β deposits and blood vessels in the upper laminae of the frontal cortex in Alzheimer’s disease. Alzheimer Dis Assoc Disord 2:378–383
Armstrong RA, Cairns NJ, Lantos PL (1999) Quantification of pathological lesions in the frontal and temporal lobe in ten patients diagnosed with Pick’s disease. Acta Neuropathol 19:64–70
Armstrong RA, Cairns NJ, Lantos PL (2000) A quantitative study of the pathological lesions in the neocortex and hippocampus of 12 patients with corticobasal degeneration. Exp Neurol 163:348–356
Armstrong RA, Lantos PL, Cairns NJ (2001a) The spatial pattern of prion protein deposits in patients with sporadic Creutzfeldt–Jacob disease. Neuropathology 21:19–24
Armstrong RA, Cairns NJ, Lantos PL (2001b) What does the study of spatial patterns tell us about the pathogenesis of neurodegenerative disorders? Neuropathology 21:1–12
Armstrong RA, Lantos PL, Cairns NJ (2007a) Progressive supranuclear palsy (PSP: a quantitative study of the pathological changes in cortical and subcortical regions of eight cases. J Neural Transm 114:1569–1577
Armstrong RA, Lantos PL, Cairns NJ (2007b) Spatial topography of the neurofibrillary tangles in cortical and subcortical regions in progressive supranuclear palsy. Parkinson Rel Disord 13:50–54
Armstrong RA, McKee AC, Stein TD, Alvarez VE, Cairns NJ (2016) A quantitative study of tau pathology in 11 cases of chronic traumatic encephalopathy. Neuropathol Appl Neurobiol. doi:10.1111/nan.12323
Bell M, Ball M (1990) Neuritic plaques and vessels of visual cortex in ageing and Alzheimer’s dementia. Neurobiol Ageing 11:359–370
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, El Abner, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman PS, Kofler J, Kukull WA, McKenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa Maria I, Seeley WW, Serramo-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso J, Vonsattel JP, White CL, Wisniewski T, Woltzer RL, Yamada M, Nelson PT (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766
De Lacoste M, White CL (1993) The role of cortical connectivity in Alzheimer’s disease pathogenesis: a review and model system. Neurobiol Aging 14:1–16
Dickson DW (2009) Neuropathology of non-Alzheimer degenerative disorders. Int J Clin Exp Pathol 3:1–23
Geddes J, Vowles G, Nicoll J, Revesz T (1999) Neuronal cytoskeletal changes are an early consequence of repetitive brain injury. Acta Neuropathol 98:171–178
Goedert M, Clavaguera F, Tolnay M (2010) The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci 33:317–325
Goldstein LE, Fisher AM, Tagge CA, ZhangXL Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletani CJ, Maglakelidze GM, Casey M, Moncaster JA, Minaeva O, Moir MD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blustajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Samans S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC (2012) Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 4:134ra60
Graham DI, Gentleman SM, Lynch A, Roberts GW (1995) Distribution of beta-amyloid protein in the brain following severe head-injury. Neuropath Appl Neurobiol 21:27–34
Hiorns RW, Neal JW, Pearson RCA, Powell TPS (1991) Clustering of ipsilateral cortico-cortical projection neurons to area 7 in the rhesus monkey. Proc R Soc (Lond) B 246:1–9
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer Dement 8:1–13
Johnson VE, Stewart W, Smith DH (2012) Widespread tau and amyloid-beta pathology many years after a single traumatic injury in humans. Brain Pathol 22:142–149
Jordan BD (2013) The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol 9:222–230
Josephs KA, Whitwell JL, Jack CR, Parisi JE, Dickson DW (2006) Frontotemporal lobar degeneration without lobar atrophy. Arch Neurol 63:1632–1638
Kawai M, Kalaria R, Harik S, Perry G (1990) The relationship of amyloid plaques to cerebral capillaries in Alzheimer’s disease. Am J Pathol 137:1435–1446
Kieman PT, Montinegro PH, Solomon TM, McKee AC (2015) Chronic traumatic encephalopathy: A neurodegenerative consequence of repetitive brain injury. Sem Neurol 35:20–28
Luthert P, Williams J (1991) A quantitative study of the coincidence of blood vessels and A4 protein deposits in Alzheimer’s disease. Neurosci Lett 126:110–112
Maroon JC, Winkelman R, Bost J, Amos A, Mathyssek C, Miele V (2015) Chronic traumatic encephalopathy in contact sports: a systematic review of all reported pathological cases. PLoS One 10:e0117338
McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE et al (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136:43–64
McKee AC, Daneshvar DH, Alvarez VE, Stein TD (2014) The neuropathology of sport. Acta Neuropathol 127:29–51
McKee AC, Stein TD, Kieman PT, Alvarez VE (2015) The neuropathology of chronic traumatic encephalopathy. Brain Pathol 25:350–364
McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, Perl D, Stein TD, Vonsattel JP, Stewart W, Tripodis Y, Crary JF, Bienick KF, Dams-O’Connor K, Alverez VF, Gordon WA, The TBI, CTE Group (2016) The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 131:75–86
Saing T, Dick M, Nelson PT, Kim RC, Cribbs DH, Head E (2012) Frontal cortex neuropathology in dementia pugilistica. J Neurotrauma 29:1054–1070
Schmidt M, Zhukareva V, Newell L, Lee V, Trojanoswki J (2001) Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer’s disease. Acta Neuropathol 101:518–524
Shetty AK, Mishra V, Kodali M, Hattiangady B (2014) Blood brain barrier dysfunction and delayed neurological deficits in mild traumatic brain injury induced by blast shock waves. Front Cell Neurosci 8:232
Stein TD, Alvarez VE, McKee AC (2014) Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimer Res Ther 6:4
Stein TD, Montenigro PH, Alvarez VE, Xia W, Crary JF, Tripodis Y, Daneshvar DH, Mez J, Soloman T, Meng G, Kubilus CA, Cormier KA, Meng KA, Babcock K, Kiernan P, Murphy L, Nowiski CK, Martin B, Dixon D, Stern RA, Cantu RC, Kowall NW, McKee AC (2015) Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol 130:21–34
Acknowledgments
The authors gratefully acknowledge the use of the resources and facilities at the Edith Nourse Rogers Memorial Veterans Hospital (Bedford, MA, USA). We also gratefully acknowledge the help of all members of the Chronic Traumatic Encephalopathy Program at Boston University School of Medicine, VA Boston, and the Bedford VA, as well as the individuals and families whose participation and contributions made this work possible. This work was supported by the National Institute of Neurological Disorders and Stroke (1U01NS086659-01), Department of Veterans Affairs, the Veterans Affairs Biorepository (CSP 501), the Translational Research Center for Traumatic Brain Injury and Stress Disorders (TRACTS), Veterans Affairs Rehabilitation Research and Development Traumatic Brain Injury Center of Excellence (B6796-C), the National Institute of Aging Boston University Alzheimer’s Disease Center (P30AG13846; supplement 0572063345–5). This work was supported by the Charles F. and Joanne Knight Alzheimer’s Disease Research Center, Washington University School of Medicine, St. Louis, MO, USA (P50 AG05681 and P01 AG03991 from the National Institute on Aging). This work was also supported by unrestricted gifts from the National Football League, Andlinger Foundation, and WWE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent was given for the removal of all brain tissue subject to local ethical committee approval and the 1996 Declaration of Helsinki (as modified Edinburgh 2000).
Conflict of interest
Other than the stated grants, the authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Armstrong, R.A., McKee, A.C., Alvarez, V.E. et al. Clustering of tau-immunoreactive pathology in chronic traumatic encephalopathy. J Neural Transm 124, 185–192 (2017). https://doi.org/10.1007/s00702-016-1635-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1635-1