Abstract
Background
Five-aminolevulinic acid (Gliolan, medac, Wedel, Germany, 5-ALA) is approved for fluorescence-guided resections of adult malignant gliomas. Case reports indicate that 5-ALA can be used for children, yet no prospective study has been conducted as of yet. As a basis for a study, we conducted a survey among certified European Gliolan users to collect data on their experiences with children.
Methods
Information on patient characteristics, MRI characteristics of tumors, histology, fluorescence qualities, and outcomes were requested. Surgeons were further asked to indicate whether fluorescence was “useful”, i.e., leading to changes in surgical strategy or identification of residual tumor. Recursive partitioning analysis (RPA) was used for defining cohorts with high or low likelihoods for useful fluorescence.
Results
Data on 78 patients <18 years of age were submitted by 20 centers. Fluorescence was found useful in 12 of 14 glioblastomas (85 %), four of five anaplastic astrocytomas (60 %), and eight of ten ependymomas grades II and III (80 %). Fluorescence was found inconsistently useful in PNETs (three of seven; 43 %), gangliogliomas (two of five; 40 %), medulloblastomas (two of eight, 25 %) and pilocytic astrocytomas (two of 13; 15 %). RPA of pre-operative factors showed tumors with supratentorial location, strong contrast enhancement and first operation to have a likelihood of useful fluorescence of 64.3 %, as opposed to infratentorial tumors with first surgery (23.1 %).
Conclusions
Our survey demonstrates 5-ALA as being used in pediatric brain tumors. 5-ALA may be especially useful for contrast-enhancing supratentorial tumors. These data indicate controlled studies to be necessary and also provide a basis for planning such a study.
Similar content being viewed by others
Introduction
Five-aminolevulinic acid (5-ALA, Gliolan) is approved for fluorescence-guided resections of malignant gliomas in Europe and a number of countries abroad. Approval of 5-ALA was based on a randomized study in adults and is now commonly used [1–10]. ALA induces the accumulation of fluorescent protoporphyrin IX (PpIX) in malignant gliomas via the heme biosynthesis pathway. This fluorochrome can be visualized intra-operatively and is useful for the identification of residual tumor. Since its approval in 2007, numerous reports have appeared also describing possible uses of 5-ALA in tumors other than gliomas, such as meningiomas, metastases, and others [11–13].
In many pediatric brain tumors, safe maximal resections have also been linked to prognosis [14–20], and it has always been of interest whether 5-ALA would be useful in this patient population. However, the histologist encountered in typical intra-axial contrast-enhancing tumors in children are much more varied than in adults. Apart from the malignant gliomas, in which 5-ALA has an established track record in adults, pediatric tumors include primitive neuroectodermal tumors, ependymomas, pilocytic astrocytomas, medulloblastomas, and others. Little is known about the extent of PpIX accumulation and fluorescence after 5-ALA administration in these childhood tumors and whether this fluorescence is actually useful for surgery, thus justifying any risks involved in 5-ALA application. No controlled studies have been performed so far. Two case reports describe useful fluorescence in a pleomorphic astrocytoma [21], the other in a medulloblastoma [22]. In addition, Preuss et al. [23] published a compilation of 18 children operated on with Gliolan in a compassionate-use setting compiled from three neurosurgical centers in Germany and France and covering a variety of pediatric brain tumor pathologies. Results were equivocal as only eight of 14 tumors displayed fluorescence, which was in turn considered to be “useful” in only seven children (three glioblastomas, one pleomorphic neuroectodermal tumor, one ependymoma III, one anaplastic astrocytoma, and one pilocytic astrocytoma). In a further small series of three case reports, useful fluorescence was found in two glioblastoma, but not in a case of medulloblastoma [24], underlining ambiguous findings. In addition, more recently, another series was published, this time discussing the use of 5-ALA in 16 children operated on at a single center outside the framework of a controlled clinical trial and with an undefined source of 5-ALA [25]. The youngest child in this series was 1 year old. Again, a variety of different pediatric tumors was observed with 5-ALA apparently inducing fluorescence in only three of 16 children (one anaplastic astrocytoma, one pilocytic astrocytoma, one glioblastoma). The question of whether this fluorescence was truly helpful was not addressed. On the other hand, distinct increases in liver enzymes in response to 5-ALA were observed, such as an increase of alanine aminotransferase (ALT). This was more evident more in younger children [25].
Especially this last series cautions that there is uncontrolled use of 5-ALA outside of clinical trials in children, despite possible side-effects and potential limitations due to the many different brain tumor types that will potentially be treated.
These observations strongly underline the necessity for a controlled clinical trial of Gliolan in children with an emphasis on safety. However, since all results so far were rather ambiguous, it appeared wise to collect further data before planning such a trial. For this reason, certified Gliolan users throughout Europe were asked to submit their anonymous data on children operated in their centers in an off-label setting. The collected information was intended to create a larger database with the aim of identifying those children pre-operatively which might benefit from 5-ALA, in order to design a future controlled study.
Methods
This retrospective survey on children and juveniles <18 years of age was based on a structured questionnaire that was distributed to Gliolan users throughout Europe who participate in the Gliolan risk management program mandated by the European Medicines Agency (EMA). This questionnaire required the following information:
-
Patient characteristics: age, sex, weight, Karnofsky Performance Score (KPS)
-
Preoperative symptoms (nausea, vomiting, seizures, ataxia, paresis, visual symptoms, other)
-
Dosing information (dose, time of application prior to anesthesia)
-
Preoperative magnetic resonance imaging (MRI) data
-
Location (intra- or extra-axial, supra- or infratentorial)
-
Degree of contrast enhancement (strong, weak/patchy, none)
-
-
Recurrence status (yes or no)
-
Preoperative symptoms
-
Final histology
-
Quality of fluorescence (strong, weak/patchy, none)
-
“Usefulness” of 5-ALA, defined as a change in surgical strategy or identification of residual tumor based on 5-ALA fluorescence
-
Resection status
-
Adverse events or lack thereof
Twenty centers provided their data. No source data verification was performed for this study.
As all children were treated in an off-label setting and this was a retrospective cohort study, ethical committee approval was not acquired from any of the participants prior to off-label administration of Gliolan. Contributing surgeons indicate having obtained informed consent in all cases. Since all participating physicians were certified in the context of the European Risk Management Plan, experience in each center was high and patients were generally treated in accordance with the general principles recommended for the use in adults, i.e., application of 20 mg/kg body weight orally and post-operative light protection for 24 h.
Data on children published in the three-center compilation by Preuss et al. [23] were included in this investigation using the structured questionnaire.
Statistics
Univariate and multivariate logistic regression analyses were performed for testing interrelationships between factors. The Chi-squared test was used for testing contingency tables of nominal data and the t test for comparing numerical data. Recursive partitioning analysis (RPA) was used for defining subgroups with/without the likelihood of having “useful” fluorescence. All statistical analyses, including RPA analysis, were performed using JMP statistics software (version 11.1.1).
Results
Children’s demographic characteristics are given in Table 1. Figure 1 details the distribution of the children’s ages. The median age was 13, the median KPS was 90, and the median weight 42.4 kg. The most common preoperative symptom was “headache” (30/78; 38.4 %), followed by “nausea/vomiting” (20/78, 25.6 %), visual symptoms (13/78, 16.7 %), “paresis” (9/78, 11.5 %), and ataxia (8/78, 10.2 %). “Other symptoms” included changes in personality, somnolence (n = 2), drowsiness, loss of fine movements of hand, nystagmus, abducens nerve paresis (n = 2), neck pain, walking disability, facial nerve paresis, anisocoria, hydrocephalus, fatigue, aphasia, and dyslexia.
All children were given 20 mg/kg Gliolan orally dissolved in tap water. However, there was a wide variation in timing of application of 5-ALA solution prior to surgery, as detailed in Fig. 2. Solutions were generally administered between 2 and 4 h prior to surgery. A distinct group of children was given solution at 6 h prior to surgery.
Apart from fluorescence, other methods for location of tumor were used intra-operatively, with neuronavigation employed in 57 of 78 (73.1 %) cases, ultrasound in 35 of 78 (44.9 %) cases, and intra-operative CT in one case. Mapping/monitoring techniques were used in 17 of 78 children (21.8 %).
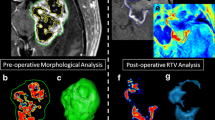
As expected, the variability of different tumors that were considered amenable to fluorescence-guided resection prior to surgery was high. The most frequent tumors were glioblastomas, followed by anaplastic astrocytomas, ependymomas grade III, PNETs, gangliogliomas, medulloblastomas, and pilocytic astrocytomas. These seven entities accounted for 59 of 78 tumors (>4 patients, Table 2). All of these tumors showed contrast enhancement to a variable extent on MRI. Fluorescence, however, was inconstant and was found to be useful in only 28 of these patients, the least commonly in medulloblastomas and pilocytic astrocytomas and inconsistently in gangliogliomas and PNETs. Among the infrequent entities found in this series (<4 patients, Table 3), all three ependymomas grade II showed useful fluorescence (Fig. 3). It was notable that the only meningiomas in this group also revealed useful fluorescence, as well as did a meningeal sarcoma.
Example of fluorescence in ependymoma grade II (female, 12 years of age). Top left: blue light image of typical red fluorescence characterized as strong and related to ependymoma tissue (Zeiss Pentero); top right: Corresponding white light image. Bottom left: preoperative MRI showing tumor of 4th ventricle with patchy contrast-enhancement; middle: blue light image of tissue specimen; right: corresponding white light image
For practical use, it would be of value to determine factors ex ante for predicting useful fluorescence before applying 5-ALA. To identify such factors, single and multiple factor nominal-logistic regression analysis was performed (Table 4). For this analysis, two children with spinal tumors (one lipoma, one pilocytic astrocytoma) were omitted. Neither a single nor a combination of multiple factors was found to significantly predict the accumulation of “useful” fluorescence. However, there was a tendency for location (supra-, infratentorial) recurrence status (recurrence yes/no) and contrast enhancement on the pre-operative MRI to predict “usefulness”.
Recursive partition analysis (RPA) was employed using these factors in order to better understand the interaction between these factors and the criterion “usefulness”. RPA generated a decision tree with five terminal nodes after three splits with different groups of patients characterized by varying likelihoods for the “usefulness” of fluorescence. Usefulness was highest in patients with tumors located supratentorially, strong contrast enhancement on MRI, and first surgery (64.3 %) as compared to patients with infratentorial tumors and first surgery (23.1 %, Fig. 4).
Decision tree generated from recursive partitioning analysis for ex ante determination of the likelihood for “usefulness”. Factors: Location; contrast enhancement on MRI, recurrence status (“useful” = provoking a change in surgical strategy or helping detect residual tumor; two children with spinal tumors excluded). There are five terminal nodes based on the likelihood of “usefulness” of fluorescence after three splits
“Complete” resections on post-operative MRI were documented in 62 patients (79.5 %; one missing). However, there was no significant relationship between fluorescence manifestation, usefulness of fluorescence, and completeness of resection.
Table 5 summarizes adverse events reported in this study. Study participants reported no toxicological adverse events and in the majority of cases, no neurological adverse events were given. However, adverse events were recorded in eight of 78 patients (Table 5). In four of these patients, either weak/patchy or strong fluorescence was found, resulting in “useful” fluorescence in only two patients, one with post-operative hygroma after resection of a grade II ependymoma, and one with transient hoarseness after resection of a grade III ependymoma of the posterior fossa. In one patient (recurrence for medulloblastoma), unspecific fluorescence was found in gliotic tissue. Unspecific fluorescence was also described in one patient with recurrent oligodendroglioma grade III. In both cases, fluorescence was characterized as weak/patchy.
Discussion
Gliolan (5-ALA) is approved for fluorescence-guided resections of malignant gliomas in Europe and other countries in adults but not in children. Thus, any use in children is strictly off-label. In this patient population, a general recommendation for the use of 5-ALA cannot be given and thus should be restricted to selected cases, since the safety profile has not been established, especially in young children. Nevertheless, there is now indication from the literature that 5-ALA appears to be used in several centers on an off-label basis despite the absence of data from controlled studies, especially on safety. In addition, the methods of application are not standardized and even in the present series the timing of administration varied widely. This is an undesirable development. Obviously, controlled studies are needed to determine both whether 5-ALA is toxicologically safe in children and whether 5-ALA is useful for facilitating detection or visualization of typical pediatric brain tumors.
In contrast to adults, the spectrum of brain tumor types is much broader in children. Not all tumor types may be good candidates for 5-ALA fluorescence-guided resections, even when they are intra-axial and show contrast enhancement on MRI. Especially pilocytic astrocytomas of the posterior fossa may be strongly enhancing and in some cases may be confused for medulloblastomas from imaging alone. Since they are benign in nature and usually well circumscribed intra-operatively, they may not be suitable for fluorescence-guided resections.
Schwake et al. [26] have demonstrated that cultures of cells derived from childhood brain tumors show differences in vitro regarding the level and the time course of PpIX accumulation. Medulloblastoma, PNET, and malignant glioma cell lines all showed variable results. Similar differential accumulation has been observed in medulloblastoma cell lines by others [27]. These observations alone question the expectation that pediatric brain tumors, even if malignant, will uniformly show useful fluorescence.
Thus, designing a study for the use of 5-ALA in children requires careful planning regarding which types of potential tumors to include. Since the available data collected and published so far are restricted, we set out to compile cases of children treated by European Gliolan users certified in the context of the Risk Management Plan imposed by EMA.
The resulting series is the largest to date with a collection of 78 children, now allowing more distinct conclusions regarding the usefulness of 5-ALA in this patient population, when 5-ALA is used in the same dosage and with comparable timing as compared to the adult population.
The results show glioblastomas and anaplastic astrocytomas to be similarly amenable to fluorescence-guided resections as in adults. It was interesting to determine that nine of ten ependymomas, regardless of whether they were grade II or III, showed fluorescence, and in eight of these cases fluorescence was considered useful for resection (Tables 2 and 3). Notably, the only child without intra-operative tumor fluorescence regurgitated the 5-ALA solution immediately after ingestion. If ependymoma is suspected, 5-ALA may be a good technical adjunct to surgery.
On the other hand, pilocytic astrocytomas only showed useful fluorescence in a minority of cases. Surprisingly, medulloblastomas, which are considered malignant, revealed useful fluorescence in only one-quarter of the eight available cases.
Although being only of academic value at this stage, the single meningiomas and meningeal sarcoma displayed useful fluorescence. Useful fluorescence in meningiomas resection has been described in several adult series [11, 13]. These observations show that “malignancy” or contrast enhancement by themselves do not represent independent factors that will reliably predict the accumulation of “useful” fluorescence in pediatric brain tumors. Also, since so far the mode of application of 5-ALA used in this series is similar to adults, it remains to be elucidated whether different dosages or timing will be more adequate for tumors that showed inconsistent fluorescence accumulation in this series, e.g., gangliogliomas or PNETs.
From a practical point of view, a histological diagnosis is generally not available prior to definite surgery (with the exception of previous biopsy). Thus, algorithms are necessary that allow ex ante predictions of the availability of useful fluorescence. From our analysis, we propose a decision tree based on recursive partition analysis. This analysis shows children with supratentorial, strongly contrast-enhancing tumors to have the greatest potential benefit from 5-ALA, if histology is unknown and if the timing and dosages that have been found useful in the adult malignant glioma population are employed.
In two patients, the occurrence of unspecific fluorescence in single samples was noted, always weak in nature and confined to gliosis. Although fluorescence is considered highly predictive in recurrent gliomas with a positive predictive value of over 95 % [28], weak fluorescence has sometimes been found in these patients and related to tissues to gliosis and scarring but not to functional brain. Similar tissue alterations cannot principally be ruled out in recurrent pediatric brain tumors.
Reported complications in this series were few and not evidently related to the use of 5-ALA, although a standardized analysis of laboratory values has not been performed. In their retrospective series of 16 children treated with 5-ALA, Beez et al. [25] reported a transient increase in liver enzymes, which has also been reported in adults [29]. In adults, this elevation is transient and has not been considered an indicator of liver damage but rather related to metabolism of 5-ALA and its downstream metabolites.
Given the present data, a future trial designed by our group in children will only consider intraaxial, strongly contrast-enhancing supratentorial tumors either in the primary or in the recurrent setting. We justify the exclusion of children with posterior fossa tumors in a context of first controlled trial by the low frequency of useful fluorescence observed in this group, especially in a first surgery setting without previous histological diagnosis. In these cases, less than 25 % of children revealed tumors with useful fluorescence due to the high incidence of pilocytic astrocytomas in patients with first surgery. This trial will primarily focus on toxicological safety and secondarily on the positive predictive value of fluorescence for detecting and visualizing tumor. The timing of administration will be uniform and earlier than found in the present study, given experimental data available so far for in vitro childhood brain tumors [26].
Such a study does not rule out the future use of 5-ALA in entities in which the accumulation of useful fluorescence is less likely, as suggested by the current data, but only on the basis of established safety data.
A future randomized study in pediatric patients with a particular tumor subtype, e.g., glioblastoma with survival as an endpoint, is unlikely to be designed due to the overall paucity of these tumors and the established use of 5-ALA in adult patients.
References
Aldave G, Tejada S, Pay E, Marigil M, Bejarano B, Idoate MA, Diez-Valle R (2013) Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic acid-guided surgery. Neurosurgery 72(6):915–20, discussion 920–921
Diez Valle R, Tejada Solis S, Idoate Gastearena MA, Garcia de Eulate R, Dominguez Echavarri P, Aristu Mendiroz J (2011) Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neuro-Oncol 102(1):105–113
Haj-Hosseini N, Richter J, Andersson-Engels S, Wardell K (2010) Optical touch pointer for fluorescence guided glioblastoma resection using 5-aminolevulinic acid. Lasers Surg Med. doi:10.1002/lsm.20868
Piccirillo SGM, Dietz S, Madhu B, Griffiths J, Price SJ, Collins VP, Watts C (2012) Fluorescence-guided surgical sampling of glioblastoma identifies phenotypically distinct tumour-initiating cell populations in the tumour mass and margin. Br J Cancer 107(3):462–468
Roessler K, Becherer A, Donat M, Cejna M, Zachenhofer I (2012) Intraoperative tissue fluorescence using 5-aminolevolinic acid (5-ALA) is more sensitive than contrast MRI or amino acid positron emission tomography ((18)F-FET PET) in glioblastoma surgery. Neurol Res 34(3):314–317
Schucht P, Beck J, Abu-Isa J, Andereggen L, Murek M, Seidel K, Stieglitz L, Raabe A (2012) Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery 71(5):927–35, discussion 935–936
Schucht P, Knittel S, Slotboom J, Seidel K, Murek M, Jilch A, Raabe A, Beck J (2014) 5-ALA complete resections go beyond MR contrast enhancement: shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir (Wien) 156(2):305–312, discussion 312
Schucht P, Murek M, Jilch A, Seidel K, Hewer E, Wiest R, Raabe A, Beck J (2013) Early re-do surgery for glioblastoma is a feasible and safe strategy to achieve complete resection of enhancing tumor. PLoS One. doi:10.1371/journal.pone.0079846
Stummer W, Tonn J-C, Goetz C, Ullrich W, Stepp H, Bink A, Pietsch T, Pichlmeier U (2014) 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 74(3):310–9, discussion 319–320
Valdes PA, Leblond F, Kim A, Harris BT, Wilson BC, Fan X, Tosteson TD, Hartov A, Ji S, Erkmen K, Simmons NE, Paulsen KD, Roberts DW (2011) Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. J Neurosurg 115(1):11–17
Della Puppa A, Rustemi O, Gioffre G, Troncon I, Lombardi G, Rolma G, Sergi M, Munari M, Cecchin D, Gardiman MP, Scienza R (2014) Predictive value of intraoperative 5-aminolevulinic acid-induced fluorescence for detecting bone invasion in meningiomas surgery. J Neurosurg 120(4):840–845
Kamp MA, Grosser P, Felsberg J, Slotty PJ, Steiger H-J, Reifenberger G, Sabel M (2012) 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir (Wien) 154(2):223–228, discussion 228
Valdes PA, Bekelis K, Harris BT, Wilson BC, Leblond F, Kim A, Simmons NE, Erkmen K, Paulsen KD, Roberts DW (2014) 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence in meningiomas: qualitative and quantitative measurements in vivo. Neurosurgery 10(Suppl 1):74–82, discussion 82–83
Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P (1996) Effects of medulloblastoma resections on outcome in children: a report from the Children’s Cancer Group. Neurosurgery 38(2):265–271
Amirian ES, Armstrong TS, Aldape KD, Gilbert MR, Scheurer ME (2012) Predictors of survival among pediatric and adult ependymoma cases: a study using surveillance, epidemiology, and end results data from 1973 to 2007. Neuroepidemiology 39(2):116–124
Fisher PG, Tihan T, Goldthwaite PT, Wharam MD, Carson BS, Weingart JD, Repka MX, Cohen KJ, Burger PC (2008) Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer 51(2):245–250
Peters O, Gnekow AK, Rating D, Wolff JEA (2004) Impact of location on outcome in children with low-grade oligodendroglioma. Pediatr Blood Cancer 43(3):250–256
Rieken S, Mohr A, Habermehl D, Welzel T, Lindel K, Witt O, Kulozik AE, Wick W, Debus J, Combs SE (2011) Outcome and prognostic factors of radiation therapy for medulloblastoma. Int J Radiat Oncol Biol Phys. doi:10.1016/j.ijrobp.2010.12.042
Song KS, Phi JH, Cho B-K, Wang K-C, Lee JY, Kim DG, Kim IH, Ahn HS, Park S-H, Kim S-K (2010) Long-term outcomes in children with glioblastoma. J Neurosurg Pediatr 6(2):145–149
Stokland T, Liu J-F, Ironside JW, Ellison DW, Taylor R, Robinson KJ, Picton SV, Walker DA (2010) A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro-Oncol 12(12):1257–1268
Ruge JR, Liu J (2009) Use of 5-aminolevulinic acid for visualization and resection of a benign pediatric brain tumor. J Neurosurg Pediatr 4(5):484–486
Eicker S, Sarikaya-Seiwert S, Borkhardt A, Gierga K, Turowski B, Heiroth H-J, Steiger H-J, Stummer W (2011) ALA-induced porphyrin accumulation in medulloblastoma and its use for fluorescence-guided surgery. Cent Eur Neurosurg 72(2):101–103
Preuss M, Renner C, Krupp W, Christiansen H, Fischer L, Merkenschlager A, Kieß W, Müller W, Manzo N, Meixensberger J, Nestler U (2013) The use of 5-aminolevulinic acid fluorescence guidance in resection of pediatric brain tumors. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 29(8):1263–1267
Barbagallo GMV, Certo F, Heiss K, Albanese V (2014) 5-ALA fluorescence-assisted surgery in pediatric brain tumors: report of three cases and review of the literature. Br J Neurosurg. doi:10.3109/02688697.2014.913779
Beez T, Sarikaya-Seiwert S, Steiger H-J, Hanggi D (2014) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of brain tumors in children–a technical report. Acta Neurochir (Wien). doi:10.1007/s00701-014-1997-9
Schwake M, Gunes D, Kochling M, Brentrup A, Schroeteler J, Hotfilder M, Fruehwald MC, Stummer W, Ewelt C (2014) Kinetics of porphyrin fluorescence accumulation in pediatric brain tumor cells incubated in 5-aminolevulinic acid. Acta Neurochir (Wien). doi:10.1007/s00701-014-2096-7
Ritz R, Scheidle C, Noell S, Roser F, Schenk M, Dietz K, Strauss WSL (2012) In vitro comparison of hypericin and 5-aminolevulinic acid-derived protoporphyrin IX for photodynamic inactivation of medulloblastoma cells. PLoS One. doi:10.1371/journal.pone.0051974
Nabavi A, Thurm H, Zountsas B, Pietsch T, Lanfermann H, Pichlmeier U, Mehdorn M (2009) Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery 65(6):1070–6, discussion 1076–1077
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. doi:10.1016/S1470-2045(06)70665-9
Conflict of interest
This study was supported logistically by medac Gesellschaft für klinische Spezialpräparate mbH, Wedel. Floriano Rodrigues is affiliated with this company. Walter Stummer has received speaker’s fees from medac. Otherwise, the authors do not have personal or institutional financial interest in 5-ALA for fluorescence-guided resections.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Stummer, W., Rodrigues, F., Schucht, P. et al. Predicting the “usefulness” of 5-ALA-derived tumor fluorescence for fluorescence-guided resections in pediatric brain tumors: a European survey. Acta Neurochir 156, 2315–2324 (2014). https://doi.org/10.1007/s00701-014-2234-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-014-2234-2