Abstract
Background
The vasodilatator effects of testosterone have been widely studied and demonstrated. Based on previous studies of these vasodilatatory activities, we hypothesized that testosterone might have potential effects on subarachnoid hemorrhage-induced cerebral vasospasm.
Methods
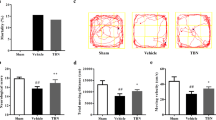
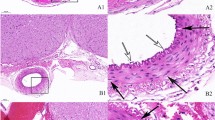
Thirty-two adult male New Zealand white rabbits were randomly divided into four groups of eight rabbits in each group: group 1 (control); group 2 (subarachnoid hemorrhage); group 3 (subarachnoid hemorrhage + vehicle); and group 4 (subarachnoid hemorrhage + testosterone). Testosterone (15 mg/kg, intraperitoneally) was administered 5 min after the intracisternal blood injection and continued for 72 h once per day in the same dose for group 4. Animals were killed 72 h after subarachnoid hemorrhage. Basilar artery cross-sectional areas, arterial wall thicknesses, and hippocampal degeneration scores were evaluated in all groups.
Results
Intraperitoneal administration of testosterone was found to attenuate cerebral vasospasm and provide neuroprotection after subarachnoid hemorrhage in rabbits. Testosterone treatment was determined to be effective at increasing the luminal area and reducing the wall thickness of the basilar artery.
Conclusions
Our findings show that testosterone has some preventive effects on SAH-induced vasospasm and secondary neuronal injury in rabbits. We propose that the vasodilatatory activity of testosterone is due to its effects on inhibiting calcium channels, activating potassium channels, augmenting nitric oxide synthesis, and inhibiting oxidant stress and inflammation.





Similar content being viewed by others
References
Dorsch NW (1995) Cerebral arterial spasm-a clinical review. Br J Neurosurg 9:403–412
Kolias AG, Sen J, Belli A (2009) Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: putative mechanisms and novel approaches. J Neurosci Res 87:1–11
Dusick JR, Gonzalez NR (2013) Management of arterial vasospasm following aneurysmal subarachnoid hemorrhage. Semin Neurol 33:488–497
Koide M, Nystoriak MA, Brayden JE, Wellman GC (2011) Impact of subarachnoid hemorrhage on local and global calcium signaling in cerebral artery myocytes. Acta Neurochir Suppl 110:145–150
Tani E, Matsumoto T (2004) Continuous elevation of intracellular Ca2+ is essential for the development of cerebral vasospasm. Curr Vasc Pharmacol 2:13–21
Białek M, Zaremba P, Borowicz KK, Czuczwar SJ (2004) Neuroprotective role of testosterone in the nervous system. Pol J Pharmacol 56:509–518
Chou TM, Sudhir K, Hutchison SJ, Ko E, Amidon TM, Collins P, Chatterjee K (1996) Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation 94:2614–2619
Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ (2001) Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol 281:H1720–H1727
English KM, Jones RD, Jones TH, Morice AH, Channer KS (2002) Testosterone acts as a coronary vasodilatator by a calcium antagonistic action. J Endocrinol Invest 25:455–458
Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collins P (1995) Testosterone relaxes rabbit coronary arteries and aorta. Circulation 91:1154–1160
Ding AQ, Stallone JN (2001) Testosterone-induced relaxation of rat aorta is androgen structure specific and involves K + channel activation. J Appl Physiol 91:2742–2750
Honda H, Unemoto T, Kogo H (1999) Different mechanisms for testosterone-induced relaxation of aorta between normotensive and spontaneously hypertensive rats. Hypertension 34:1232–1236
Tep-areenan P, Kendall DA, Randall MD (2002) Testosterone-induced vasorelaxation in the rat mesenteric arterial bed is mediated predominantly via potassium channels. Br J Pharmacol 135:735–740
Jones RD, English KM, Pugh PJ, Morice AH, Jones TH, Channer KS (2002) Pulmonary vasodilatatory action of testosterone: evidence of a calcium antagonistic action. J Cardiovasc Pharmacol 39:814–823
Jones RD, Pugh PJ, Jones TH, Channer KS (2003) The vasodilatatory action of testosterone: a potassium-channel opening or a calcium antagonistic action? Br J Pharmacol 138:733–744
Seyrek M, Yildiz O, Ulusoy HB, Yildirim V (2007) Testosterone relaxes isolated human radial artery by potassium channel opening action. J Pharmacol Sci 103:309–316
Ramírez-Rosas MB, Cobos-Puc LE, Muñoz-Islas E, González-Hernández A, Sánchez-López A, Villalón CM, Maassenvandenbrink A, Centurión D (2011) Pharmacological evidence that Ca2+ channels and, to a lesser extent, K + channels mediate the relaxation of testosterone in the canine basilar artery. Steroids 76:409–415
Lu Y, Fu Y, Ge Y, Juncos LA, Reckelhoff JF, Liu R (2012) The vasodilatatory effect of testosterone on renal afferent arterioles. Gend Med 9:103–111
Perusquía M, Stallone JN (2010) Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol 298:H1301–H1307
Chen Z, Xi G, Mao Y, Keep RF, Hua Y (2011) Effects of progesterone and testosterone on ICH-induced brain injury in rats. Acta Neurochir Suppl 111:289–293
Oloyo AK, Sofola OA, Anigbogu CN, Nair RR, Vijayakumar HS, Fernandez AC (2013) Testosterone reduces vascular relaxation by altering cyclic adenosine monophosphate pathway and potassium channel activation in male Sprague Dawley rats fed a high-salt diet. Ther Adv Cardiovasc Dis 7:75–85
Kertmen H, Gürer B, Yilmaz ER, Arikok AT, Kanat MA, Ergüder BI, Sekerci Z (2014) The comparative effects of recombinant human erythropoietin and darbepoetin-alpha on cerebral vasospasm following experimental subarachnoid hemorrhage in the rabbit. Acta Neurochir (Wien) 156:951–962
Bederson JB, Connolly ES Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE Jr, Harbaugh RE, Patel AB, Rosenwasser RH (2009) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 40:994–1025
Kertmen H, Gürer B, Yilmaz ER, Arikok AT, Demirci A, Gökyaprak SM, Sekerci Z (2012) The effect of thiocolchicoside on cerebral vasospasm following experimental subarachnoid hemorrhage in the rabbit. Acta Neurochir (Wien) 154:1431–1436
Iqbal MJ, Dalton M, Sawers RS (1983) Binding of testosterone and oestradiol to sex hormone binding globulin, human serum albumin and other plasma proteins: evidence for non-specific binding of oestradiol to sex hormone binding globulin. Clin Sci (Lond) 64:307–314
Ong PJ, Patrizi G, Chong WC, Webb CM, Hayward CS, Collins P (2000) Testosterone enhances flow-mediated brachial artery reactivity in men with coronary artery disease. Am J Cardiol 85:269–272
Kang SM, Jang Y, Ji K, Chung N, Cho SY, Chae JS, Lee JH (2002) Effect of oral administration of testosterone on brachial arterial vasoreactivity in men with coronary artery disease. Am J Cardiol 89:862–864
Clapham DE (1995) Calcium signaling. Cell 80:259–268
Nelson MT, Patlak JB, Worley JF, Standen NB (1990) Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol 259:C3–C18
Hai CM, Murphy RA (1989) Ca2+, crossbridge phosphorylation, and contraction. Annu Rev Physiol 51:285–298
Knot HJ, Nelson MT (1998) Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508:199–209
Ohkuma H, Ogane K, Tanaka M, Suzuki S (2001) Assessment of cerebral microcirculatory changes during cerebral vasospasm by analyzing cerebral circulation time on DSA images. Acta Neurochir Suppl 77:127–130
Takeuchi H, Handa Y, Kobayashi H, Kawano H, Hayashi M (1991) Impairment of cerebral autoregulation during the development of chronic cerebral vasospasm after subarachnoid hemorrhage in primates. Neurosurgery 28:41–48
Alvarez E, Cairrão E, Morgado M, Morais C, Verde I (2010) Testosterone and cholesterol vasodilation of rat aorta involves L-type calcium channel inhibition. Adv Pharmacol Sci 2010:534184
Crews JK, Khalil RA (1999) Antagonistic effects of 17 beta-estradiol, progesterone, and testosterone on Ca2+ entry mechanisms of coronary vasoconstriction. Arterioscler Thromb Vasc Biol 19:1034–1040
Hall J, Jones RD, Jones TH, Channer KS, Peers C (2006) Selective inhibition of L-type Ca2+ channels in A7r5 cells by physiological levels of testosterone. Endocrinology 147:2675–2680
Murphy JG, Khalil RA (1999) Decreased [Ca(2+)](i) during inhibition of coronary smooth muscle contraction by 17beta-estradiol, progesterone, and testosterone. J Pharmacol Exp Ther 291:44–52
Perusquía M, Villalón CM (1999) Possible role of Ca2+ channels in the vasodilating effect of 5beta-dihydrotestosterone in rat aorta. Eur J Pharmacol 371:169–178
Scragg JL, Jones RD, Channer KS, Jones TH, Peers C (2004) Testosterone is a potent inhibitor of L-type Ca(2+) channels. Biochem Biophys Res Commun 318:503–506
Scragg JL, Dallas ML, Peers C (2007) Molecular requirements for L-type Ca2+ channel blockade by testosterone. Cell Calcium 42:11–15
Montaño LM, Calixto E, Figueroa A, Flores-Soto E, Carbajal V, Perusquía M (2008) Relaxation of androgens on rat thoracic aorta: testosterone concentration-dependent agonist/antagonist L-type Ca2+ channel activity, and 5beta-dihydrotestosterone restricted to L-type Ca2+ channel blockade. Endocrinology 149:2517–2526
Jones RD, English KM, Jones TH, Channer KS (2004) Testosterone-induced coronary vasodilatation occurs via a non-genomic mechanism: evidence of a direct calcium antagonism action. Clin Sci (Lond) 107:149–158
Harder DR, Dernbach P, Waters A (1987) Possible cellular mechanism for cerebral vasospasm after experimental subarachnoid hemorrhage in the dog. J Clin Invest 80:875–880
Jahromi BS, Aihara Y, Ai J, Zhang ZD, Nikitina E, Macdonald RL (2008) Voltage-gated K + channel dysfunction in myocytes from a dog model of subarachnoid hemorrhage. J Cereb Blood Flow Metab 28:797–811
Koide M, Penar PL, Tranmer BI, Wellman GC (2007) Heparin-binding EGF-like growth factor mediates oxyhemoglobin-induced suppression of voltage-dependent potassium channels in rabbit cerebral artery myocytes. Am J Physiol Heart Circ Physiol 293:H1750–H1759
Cairrão E, Alvarez E, Santos-Silva AJ, Verde I (2008) Potassium channels are involved in testosterone-induced vasorelaxation of human umbilical artery. Naunyn Schmiedeberg’s Arch Pharmacol 376:375–383
Yildiz O, Seyrek M, Gul H, Un I, Yildirim V, Ozal E, Uzun M, Bolu E (2005) Testosterone relaxes human internal mammary artery in vitro. J Cardiovasc Pharmacol 45:580–585
Pluta RM, Thompson BG, Dawson TM, Snyder SH, Boock RJ, Oldfield EH (1996) Loss of nitric oxide synthase immunoreactivity in cerebral vasospasm. J Neurosurg 84:648–654
Albayrak Y, Halici Z, Odabasoglu F, Unal D, Keles ON, Malkoc I, Oral A, Yayla M, Aydin O, Unal B (2011) The effects of testosterone on intestinal ischemia/reperfusion in rats. J Invest Surg 24:283–291
Asirvatham AJ, Schmidt M, Gao B, Chaudhary J (2006) Androgens regulate the immune/inflammatory response and cell survival pathways in rat ventral prostate epithelial cells. Endocrinology 147:257–271
Vignozzi L, Cellai I, Santi R, Lombardelli L, Morelli A, Comeglio P, Filippi S, Logiodice F, Carini M, Nesi G, Gacci M, Piccinni MP, Adorini L, Maggi M (2012) Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol 214:31–43
Chisu V, Manca P, Lepore G, Gadau S, Zedda M, Farina V (2006) Testosterone induces neuroprotection from oxidative stress. Effects on catalase activity and 3-nitro-L-tyrosine incorporation into alpha-tubulin in a mouse neuroblastoma cell line. Arch Ital Biol 144:63–73
Fargo KN, Foecking EM, Jones KJ, Sengelaub DR (2009) Neuroprotective actions of androgens on motoneurons. Front Neuroendocrinol 30:130–141
Tirassa P, Thiblin I, Agren G, Vigneti E, Aloe L, Stenfors C (1997) High-dose anabolic androgenic steroids modulate concentrations of nerve growth factor and expression of its low affinity receptor (p75-NGFr) in male rat brain. J Neurosci Res 47:198–207
Kujawa KA, Emeric E, Jones KJ (1991) Testosterone differentially regulates the regenerative properties of injured hamster facial motoneurons. J Neurosci 11:3898–3906
Ahlbom E, Prins GS, Ceccatelli S (2001) Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res 892:255–262
Pike CJ (2001) Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res 919:160–165
Sowers MF, Beebe JL, McConnell D, Randolph J, Jannausch M (2001) Testosterone concentrations in women aged 25–50 years: associations with lifestyle, body composition, and ovarian status. Am J Epidemiol 153:256–264
Travison TG, Araujo AB, O’Donnell AB, Kupelian V, McKinlay JB (2007) A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab 92:196–202
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
This remains an observational rather than mechanistic study, but is well conceived and conducted. It provides hypothesis-generating data, and as such a contribution to the literature.
Michael Tymianski
Toronto, Canada
Rights and permissions
About this article
Cite this article
Gürer, B., Turkoglu, E., Kertmen, H. et al. Attenuation of cerebral vasospasm and secondary injury by testosterone following experimental subarachnoid hemorrhage in rabbit. Acta Neurochir 156, 2111–2120 (2014). https://doi.org/10.1007/s00701-014-2211-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-014-2211-9