Abstract
Background
Akt plays an important role in cell survival, proliferation, apoptosis and other activities. It also has been involved in maintaining smooth muscle cell contraction phenotypes in vitro and in vivo. Recent studies have focused on the inhibition of Akt in acute vasospasm and neuronal apoptosis after subarachnoid hemorrhage (SAH). However, its role in delayed cerebral vasospasm (DCVS) has not been reported.
Methods
In this study, using a “two-hemorrhage” rat model of SAH, we examined the expression of p-Akt and the formation of vasospasm in the basilar arteries. To investigate the possible role of Akt in phenotypic switching, we performed immunohistochemical staining to examine expressions of SMα-actin and proliferating cell nuclear antigen (PCNA), markers of smooth muscle phenotypic switching.
Results
We found that the basilar arteries exhibited vasospasm after SAH and that vasospasm became most severe on day 7 after SAH. Elevated protein expression of p-Akt was detected 4 days after SAH induction, peaked on day 7, and recovered on day 21, which was in a parallel time course to the development of DCVS. Moreover, results of immunohistochemical staining revealed enhanced expression of PCNA but gradual reduction in expression of SMα-actin from day 1 to day 7 after SAH; then, the expressions of PCNA and SMα-actin gradually recovered until day 21.
Conclusions
These results support a novel mechanism in which the Akt signaling pathway plays an important role in the proliferation of smooth muscle cells (SMCs) rather than inducing phenotype switching in basilar arteries, which promotes the development of DCVS after SAH.





Similar content being viewed by others
References
Alford PW, Dabiri BE, Goss JA, Hemphill MA, Brigham MD, Parker KK (2011) Blast-induced phenotypic switching in cerebral vasospasm. Proc Natl Acad Sci USA 108:12705–12710
Endo H, Nito C, Kamada H, Yu FS, Chan PH (2006) Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of Akt/glycogen synthase kinase-3β survival signaling. J Cerebr Blood F Met 27:975–982
Endo H, Nito C, Kamada H, Yu F, Chan PH (2006) Akt/GSK3beta survival signaling is involved in acute brain injury after subarachnoid hemorrhage in rats. Stroke 37:2140–2146
Gao C, Wang CL, Wu P, Liu Z, Liu XZ (2010) Simvastatin activates Akt/glycogen synthase kinase-3 [beta] signal and inhibits caspase-3 activation after experimental subarachnoid hemorrhage. Vasc Pharmacol 52:77–83
Kawahara S, Umemoto S, Tanaka M, Umeji K, Matsuda S, Kubo M, Matsuzaki M (2005) Up-regulation of Akt and eNOS induces vascular smooth muscle cell differentiation in hypertension in vivo. J Cardiovasc Pharm 45:367
Kawai-Kowase K, Owens GK (2007) Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am J Physiol Cell Physiol 292:C59–C69
Kim JS, Kim IK, Lee SY, Song BW, Cha MJ, Song H, Choi E, Lim S, Ham O, Jang Y, Hwang KC (2012) Anti-proliferative effect of rosiglitazone on angiotensin II-induced vascular smooth muscle cell proliferation is mediated by the mTOR pathway. Cell Biol Int 36:305–310
Nagai R, Kuro-o M, Babij P, Periasamy M (1989) Identification of two types of smooth muscle heavy chain isoforms by cDNA cloning and immunoblot analysis. J Biol Chem 264:9734–9737
Ogawa A, Firth AL, Smith KA, Maliakal MV, Yuan JX (2012) PDGF enhances store-operated Ca2+ entry by upregulating STIM1/Orai1 via activation of Akt/mTOR in human pulmonary arterial SMCs. Am J Physiol Cell Physiol 302:C405–C411
Ohkuma H, Suzuki S, Ogane K (2003) Phenotypic modulation of smooth muscle cells and vascular remodeling in intraparenchymal small cerebral arteries after canine experimental subarachnoid hemorrhage. Neurosci Lett 344:193–196
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809
Santhanam AVR, Smith LA, Akiyama M, Rosales AG, Bailey KR, Katusic ZS (2005) Role of endothelial NO synthase phosphorylation in cerebrovascular protective effect of recombinant erythropoietin during subarachnoid hemorrhage-induced cerebral vasospasm. Stroke 36:2731–2737
Song JN, Wang WB, Sui L (2010) Neurotransmitter regulation of extracellular signal-regulated kinase expression following subarachnoid hemorrhage. Neural Regen Res 5:214–220
Sugawara T, Ayer R, Jadhav V, Chen WQ, Tsubokawa T, Zhang JH (2008) Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J Neurosci Res 86:3635–3643
Taher TEI, Derksen PWB, de Boer OJ, Spaargaren M, Teeling P, van der Wal AC, Pals ST (2002) Hepatocyte growth factor triggers signaling cascades mediating vascular smooth muscle cell migration. Biochem Bioph Res Co 298:80–86
Yamaguchi-Okada M, Nishizawa S, Koide M, Nonaka Y (2005) Biomechanical and phenotypic changes in the vasospastic canine basilar artery after subarachnoid hemorrhage. J Appl Physiol 99:2045–2052
Zhang W, Khatibi NH, Yamaguchi-Okada M, Yan J, Chen C, Hu Q, Meng H, Han H, Liu S, Zhou C (2012) Mammalian target of rapamycin (mTOR) inhibition reduces cerebral vasospasm following a subarachnoid hemorrhage injury in canines. Exp Neurol 233:799–806
Zhang Z, Wang M, Fan XH, Chen JH, Guan YY, Tang YB (2012) Upregulation of TRPM7 channels by angiotensin II triggers phenotypic switching of vascular smooth muscle cells of ascending aorta. Circ Res 111:1137–1146
Zhou RH, Lee TS, Tsou TC, Rannou F, Li YS, Chien S, Shyy JYJ (2003) Stent implantation activates Akt in the vessel wall role of mechanical stretch in vascular smooth muscle cells. Arterioscl Throm Vas 23:2015–2020
Zubkov AY, Tibbs RE, Clower B, Ogihara K, Aoki K, Zhang JH (2002) Morphological changes of cerebral arteries in a canine double hemorrhage model. Neurosci Lett 326:137–141
Acknowledgments
This study was supported by the National 863 Project of China, No. 2006AA02Z4Z4; the National Natural Science Foundation of China, No. 30870844; the New Century Excellent Talent Support Project of Ministry of Education, No. NCET-05-0831; the “13115” Special Fund for Major Science and Technology Projects of Shaanxi Province, No. 2008ZDKG-66.
Author contributions
J.N. Song conceived and designed the research, and was in charge of manuscript authorization. G.S. Hao performed the research. J.Y. An wrote the manuscript. D.D. Li, P. Sun, Y. Li and J.G. Xue participated in this study.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
Unlike neuroprotective agents such as nimodipine and simvastatin, which attenuate the cellular cascade of neuronal apoptosis, an agent that effectively treats DCVS, though highly sought, has yet to be discovered. This study contributes to a body of literature intended to reveal the cellular mechanisms of cerebral vasospasm in order to identify therapeutic targets.
The premise of the study is that a phenotype switch from “contractile” to “proliferative” occurs in cerebral artery smooth muscle cells after subarachnoid hemorrhage. Similar to coronary artery disease and myocardial infarction, the proliferative smooth muscle phenotype induces synthesis of a disorganized smooth muscle matrix on the luminal side of cerebral arteries resulting in wall thickening, stenosis, reduced compliance and ultimately delayed cerebral ischemic vasospasm (DCVS).
P-Akt is a signaling protein implicated in neuroprotection after brain injury by inhibition of apoptosis and promotion of cell survival. Simvastatin, for example, is believed to employ its neuroprotective effects via phosphorylation of the Ask-GSK3 complex and inhibition of apoptotic caspase-3 proteins (1).
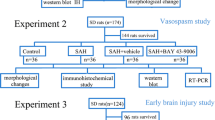
To create a model of aneurysmal subarachnoid hemorrhage (SAH), autologous blood is injected into the cisterna magna of 30 rats at zero and 48 hours. Six of the rats did not survive the injection, leaving them a population of 24. They sacrifice the animals on days 1, 4, 7, 14 and 21 in order to section the basilar arteries and measure thickness of vessel walls and perimeters of arterial lumen. Finally, they immunohistochemically stain the arterial wall for three proteins: Akt, smooth muscle alpha-actin (SMa-actin, a marker for contractile smooth muscle phenotype), and proliferating cell nuclear antigen (PCNA, a marker for proliferative smooth muscle phenotype.
They demonstrate with arterial stains and histograms that expression of SMa-actin and arterial perimeter significantly decrease on day 1, nadir on day 7, and return to baseline on day 21. Conversely, they show expression of PCNA and arterial wall thicknesses significantly increase on day 1, peak on day 7 and return to baseline on day 21. However, the novel finding here is Akt expression did not change on day 1, increased on day 4, peaked on day 7, and returned to baseline on day 21.
The fact that no increase in Akt expression occurred until four days after SAH and the increased expression of PCNA is an important finding. This suggests Akt plays a minimal role in the phenotype switch of smooth muscle. Rather, Akt appears to promote the proliferative phase after phenotype switch has occurred. Whether this new understanding of Akt’s role in DCVS increases or decreases its value as a therapeutic target remains to be determined.
As the authors point out, m-TOR is a downstream protein from Akt. The m-TOR inhibitor rapamycin has shown promise in attenuation of cerebral vasospasm (2). At this time, Akt’s susceptibility as a therapeutic target to inhibit the proliferation of smooth cells and the cascade of events leading to cerebral vasospasm has yet to be seen. We commend the authors for their systematic demonstration of the timing of Akt expression during the 21 day vasospasm window in this animal model of DCVS and agree further research on the proliferative phase of smooth muscle expression following SAH may yield beneficial therapeutic targets.
Dustin Hayward
Christopher Loftus
Illinois, USA
(1) Gao C, Wang CL, Wu P, Liu Z, Liu XZ (2010) Simvastatin activates Akt/glycogen synthase kinase-3 [beta] signal and inhibits caspase-3 activation after experimental subarachnoid hemorrhage. Vasc Pharmacol 52:77–83.
(2) Zhang W, Khatibi NH, Yamaguchi-Okada M, Yan J, Chen C, Hu Q, Meng H, Han H, Liu S, Zhou C (2012) Mammalian target of rapamycin (mTOR) inhibition reduces cerebral vasospasm following a subarachnoid hemorrhage injury in canines. Exp Neurol
Rights and permissions
About this article
Cite this article
Song, JN., An, JY., Hao, GS. et al. Role of Akt signaling pathway in delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Acta Neurochir 155, 2063–2070 (2013). https://doi.org/10.1007/s00701-013-1808-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1808-8