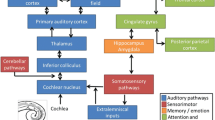
Abstract
Background
Hemifacial spasm patients often suffer from non-motor symptoms such as tinnitus. These non-motor symptoms are known to be associated with changes in cortical activity. Magnetoencephalography (MEG) is a technique that can record brain activity noninvasively. To determine the usefulness of MEG in assessing changes in cortical activity associated with non-motor hearing symptoms in hemifacial spasm patients.
Methods
We used MEG to evaluate the reactivity of the auditory cortex in 26 hemifacial spasm patients. We divided patients into a subjective tinnitus group (n = 10) and a non-tinnitus group (n = 16). The latency and amplitude of the most prominent deflection, N100m, was compared between the two groups.
Results
There was a significant difference in the pure tone audiogram on the spasm side compared with the non-spasm side. After stimulation on the spasm side, the amplitude of the N100m peak in the contralateral hemisphere was lower in the subjective tinnitus group than in the non-tinnitus group.
Conclusions
Our results indicate that MEG can detect differences in cortical activity between hemifacial spasm patients with and without tinnitus. This suggests that MEG can identify changes in cortical activity associated with non-motor symptoms.


Similar content being viewed by others
References
De Ridder D, De Mulder G, Verstraeten E, Seidman M, Elisevich K, Sunaert S, Kovacs S, Van der Kelen K, Van de Heyning P, Moller A (2007) Auditory cortex stimulation for tinnitus. Acta Neurochir Suppl 97:451–462
De Ridder D, De Mulder G, Verstraeten E, Van der Kelen K, Sunaert S, Smits M, Kovacs S, Verlooy J, Van de Heyning P, Moller AR (2006) Primary and secondary auditory cortex stimulation for intractable tinnitus. ORL J Otorhinolaryngol Relat Spec 68:48–54, discussion 54–45
Eggermont JJ, Roberts LE (2004) The neuroscience of tinnitus. Trends Neurosci 27:676–682
Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E (1995) Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375:482–484
Fregni F, Marcondes R, Boggio PS, Marcolin MA, Rigonatti SP, Sanchez TG, Nitsche MA, Pascual-Leone A (2006) Transient tinnitus suppression induced by repetitive transcranial magnetic stimulation and transcranial direct current stimulation. Eur J Neurol 13:996–1001
Gardner WJ (1968) Trigeminal neuralgia. Clin Neurosurg 15:1–56
Harrison RV, Ibrahim D, Mount RJ (1998) Plasticity of tonotopic maps in auditory midbrain following partial cochlear damage in the developing chinchilla. Exp Brain Res 123:449–460
Kaas JH (1991) Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci 14:137–167
Kaltenbach JA, Afman CE (2000) Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res 140:165–172
Kleinjung T, Eichhammer P, Langguth B, Jacob P, Marienhagen J, Hajak G, Wolf SR, Strutz J (2005) Long-term effects of repetitive transcranial magnetic stimulation (rTMS) in patients with chronic tinnitus. Otolaryngol Head Neck Surg 132:566–569
Kuroki A, Moller AR (1994) Facial nerve demyelination and vascular compression are both needed to induce facial hyperactivity: a study in rats. Acta Neurochir (Wien) 126:149–157
Lee Y, Kwon H, Kim J, Park Y, Park J (1998) Double relaxation oscillation SQUID with high flux-to-voltage transfer and its application to a biomagnetic multichannel system. J Korean Phys Soc 32:600–605
Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ (2005) Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 28:325–333
Londero A, Langguth B, De Ridder D, Bonfils P, Lefaucheur JP (2006) Repetitive transcranial magnetic stimulation (rTMS): a new therapeutic approach in subjective tinnitus? Neurophysiol Clin 36:145–155
Martinelli P, Giuliani S, Ippoliti M (1992) Hemifacial spasm due to peripheral injury of facial nerve: a nuclear syndrome? Mov Disord 7:181–184
Moller AR (1991) Interaction between the blink reflex and the abnormal muscle response in patients with hemifacial spasm: results of intraoperative recordings. J Neurol Sci 101:114–123
Moller AR, Jannetta PJ (1984) On the origin of synkinesis in hemifacial spasm: results of intracranial recordings. J Neurosurg 61:569–576
Moller MB, Moller AR (1985) Loss of auditory function in microvascular decompression for hemifacial spasm. Results in 143 consecutive cases. J Neurosurg 63:17–20
Muhlnickel W, Elbert T, Taub E, Flor H (1998) Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A 95:10340–10343
Nielsen VK (1984) Pathophysiology of hemifacial spasm: I. Ephaptic transmission and ectopic excitation. Neurology 34:418–426
Norena A, Micheyl C, Chery-Croze S, Collet L (2002) Psychoacoustic characterization of the tinnitus spectrum: implications for the underlying mechanisms of tinnitus. Audiol Neurootol 7:358–369
Plewnia C, Bartels M, Gerloff C (2003) Transient suppression of tinnitus by transcranial magnetic stimulation. Ann Neurol 53:263–266
Rajan R, Irvine DR (1998) Neuronal responses across cortical field A1 in plasticity induced by peripheral auditory organ damage. Audiol Neurootol 3:123–144
Ramirez RR, Kopell BH, Butson CR, Gaggl W, Friedland DR, Baillet S (2009) Neuromagnetic source imaging of abnormal spontaneous activity in tinnitus patient modulated by electrical cortical stimulation. Conf Proc IEEE Eng Med Biol Soc 2009:1940–1944
Rauschecker JP (1999) Auditory cortical plasticity: a comparison with other sensory systems. Trends Neurosci 22:74–80
Robertson D, Irvine DR (1989) Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol 282:456–471
Rudzinska M, Wojcik M, Szczudlik A (2010) Hemifacial spasm non-motor and motor-related symptoms and their response to botulinum toxin therapy. J Neural Transm 117:765–772
Ryan AF, Woolf NK (1988) Development of tonotopic representation in the Mongolian gerbil: a 2-deoxyglucose study. Brain Res 469:61–70
Ryu H, Yamamoto S, Sugiyama K, Nozue M (1998) Neurovascular compression syndrome of the eighth cranial nerve. What are the most reliable diagnostic signs? Acta Neurochir (Wien) 140:1279–1286
Sakaki T, Morimoto T, Miyamoto S, Kyoi K, Utsumi S, Hyo Y (1987) Microsurgical treatment of patients with vestibular and cochlear symptoms. Surg Neurol 27:141–146
Virtanen J, Ahveninen J, Ilmoniemi RJ, Naatanen R, Pekkonen E (1998) Replicability of MEG and EEG measures of the auditory N1/N1m-response. Electroencephalogr Clin Neurophysiol 108:291–298
Wang A, Jankovic J (1998) Hemifacial spasm: clinical findings and treatment. Muscle Nerve 21:1740–1747
Weisz N, Dohrmann K, Elbert T (2007) The relevance of spontaneous activity for the coding of the tinnitus sensation. Prog Brain Res 166:61–70
Wilkins RH (1991) Hemifacial spasm: a review. Surg Neurol 36:251–277
Wuertenberger CJ, Rosahl SK (2009) Vertigo and tinnitus caused by vascular compression of the vestibulocochlear nerve, not intracanalicular vestibular schwannoma: review and case presentation. Skull Base 19:417–424
Yap L, Pothula VB, Lesser T (2008) Microvascular decompression of cochleovestibular nerve. Eur Arch Otorhinolaryngol 265:861–869
Acknowledgements
We would like to thank Eun Jeong Kwon, RN, Sang Keum Park, RN, and Ester PohelKing, MD, for their assistance in preparing manuscript. This study was supported by a faculty research grant from Yonsei University College of Medicine (6-2009-0156).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, Y.S., Kim, B.S., Lee, D.K. et al. Assessment of non-motor hearing symptoms in hemifacial spasm using magnetoencephalography. Acta Neurochir 154, 509–515 (2012). https://doi.org/10.1007/s00701-011-1231-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-1231-y