Abstract
A photoelectrochemical sensor for FAD and FMN was fabricated by incorporating the reduced flavin cofactor as an electron donor into a photoelectrochemical reaction in a ZnO nanorod photoelectrode (ZnO-NR-PhE). The assay is based on the pre-reduction of the flavin coenzyme on a second working electrode. The ZnO-NR-PhE was electrochemically preparated, and synthesis was optimized. The mechanism of the reaction of flavin coenzyme on the ZnO-NR-PhE was studied. Under optimized conditions (a pH value of 6.5; a pre-reduction potential of −0.40 V; illumination at 365 nm; a light energy of 1.6 mW∙cm−2), the photocurrent at a bias voltage of 0.1 V is proportional to the logarithm of the coenzyme concentration in the range from 10 nmol⋅L−1 to 1.0 μmol⋅L−1. The detection sensitivity is 193.5 nA/logC (μmol⋅L−1) for FMN, and 195.6 nA/logC (μmol⋅L−1) for FAD. The respective detection limits are 8.0 and 5.0 nmol⋅L−1 (at an S/N ratio of 3), respectively. Compared to other methods, the one presented here has a wide analytical range, high sensitivity, and convenient operation. Common biochemical substances do not interfere.

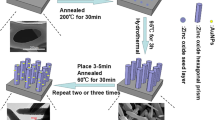
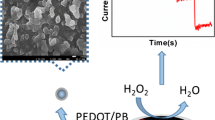
Schematic of a photoelectrochemical sensor for FAD and FMN that was was fabricated by incorporating the reduced flavin cofactor as an electron donor into a photoelectrochemical reaction in a ZnO nanorod photoelectrode.









Similar content being viewed by others
References
Zhang X, Guo Y, Liu M, Zhang S (2013) Photoelectrochemically active species and photoelectrochemical biosensors. RSC Adv 9:2846–2857
Zhao C, Yu J, Zhao G, Jiao K (2011) Fabrication of poly(thionine)/H2O2 photoelectrochemical sensing platform and determination for catalytic activity of glucose oxidase. Scientia Sinica Chim 41:1075–1080
Xu R, Jiang Y, Xia L, Zhang T, Xu L, Zhang S, Liu D, Song H (2015) A sensitive photoelectrochemical biosensor rfor AFP detection based on ZnO inverse opal electrodes with signal amplification of CdS-QDs. Biosens Bioelectron 74:411–417
Wang G-L, Shu J-X, Dong Y-M, Wu X-M, Zhao W-W, Xu J-J, Chen H-Y (2015) Using G-quadruplex/hemin to “switch-on” the cathodic photocurrent of p-type PbS quantum dots: toward a versatile platform for photoelectrochemical aptasensing. Anal Chem 87:2892–2900
Long Y-T, Kong C, Li D-W, Li Y, Chowdhury S, Tian H (2011) Ultrasensitive determination of cysteine based on the photocurrent of nafion-functionalized CdS–MV quantum dots on an ITO electrode. Small 7:1624–1628
Zhang Z, Zhao C (2013) Progress in the studies on photoelectrochemical analysis and sensors. Chinese J Anal Chem 41:436–444
Abbas CA, Sibirny AA (2011) Genetic control of biosynthesis and transport of riboflavin and flavin nucleotides and construction of robust biotechnological producers. Microbiol Mol Biol Rev 75:321–360
Lienhart WD, Gudipati V, Macheroux P (2013) The human flavoproteome. Arch Biochem Biophys 535:150–162
Barile M, Giancaspero TA, Brizio C, Panebianco C, Indiveri C, Galluccio M, Vergani L, Eberini I, Gianazza E (2013) Biosynthesis of flavin cofactors in man: implications in health and disease. Curr Pharm Des 19:2649–2675
Arya SK, Saha S, Ramirez-Vick JE, Gupta V, Bhansali S, Singh SP (2012) Recent advances in ZnO nanostructures and thin films for biosensor applications: review. Anal Chim Acta 737:1–21
Zhai Y, Zhai S, Chen G, Zhang K, Yue Q, Wang L, Liu J, Jia J (2012) Effects of morphology of nanostructured ZnO on direct electrochemistry and biosensing properties of glucose oxidase. J Electroanal Chem 656:198–205
Kang Z, Gu Y, Yan X, Bai Z, Liu Y (2015) Enhanced photoelectrochemical property of ZnO nanorods array synthesized on reduced graphene oxide for self-powered biosensing application. Biosens Bioelectron 64:499–504
Weng J, Zhang Y, Han G, Zhang Y, Xu J, Huang X, Chen K (2005) Electrochemical deposition and characterization of wide band semiconductor ZnO thin film. Thin Solid Films 478:25–29
Hambali N. A., Yahaya H., Mahmood M. R., Terasako T., Hashim A. M (2014) Synthesis of zinc oxide nanostructures on graphene/glass substrate by electrochemical deposition: effects of current density and temperature. Nanoscale Res Lett 9: 609–616
Zhou T-Y, Yu J-S (1998) Thin-layer Spectrofluoroelectrochemical studies of riboflavin. Chem J Chinese Univ 19:204–206
Hühner J, Ingles-Prieto A, Neusüß C, Lämmerhofer M, Janovjak H (2015) Quantification of riboflavin, flavin mononucleotide, and flavin adenine dinucleotide in mammalian model cells by CE with LED-induced fluorescence detection. Electrophoresis 36(4):518–525
Cataldia TR, Nardielloa D, Benedettob GE, Bufoc SA (2002) Optimizing separation conditions for riboflavin, flavin mononucleotide and flavin adenine dinucleotide in capillary zone electrophoresis with laser-induced fluorescence detection. J Chromatogr A 968:229–239
Kang C, Wu HL, Zhou C, Xiang SX, Zhang XH, Yu YJ, Yu RQ (2016) Quantitative fluorescence kinetic analysis of NADH and FAD in human plasma using three- and four-way calibration methods capable of providing the second-order advantage. Anal Chim Acta 910:36–44
Andrés-Lacueva C, Mattivi F, Tonon D (1998) Determination of riboflavin, flavin mononucleotide and flavin-adenine dinucleotide in wine and other beverages by high-performance liquid chromatography with fluorescence detection. J Chromatogr A 823:355–363
Hou W, Wang E (1990) Detection of flavins by liquid chromatography using an electrochemical detector with two electrodes in series. Analy Chim Acta 239:29–33
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No: 21475072) and by the Open-end Fund of Key Laboratory of Sensor Analysis of Tumor Marker, Ministry of Education (No. SATM201503).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zhao, C., Liu, L., Ge, J. et al. Ultrasensitive determination for flavin coenzyme by using a ZnO nanorod photoelectrode in a four-electrode system. Microchim Acta 184, 2333–2339 (2017). https://doi.org/10.1007/s00604-017-2230-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2230-3