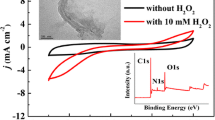
Abstract
A sandwich structured nanocomposite consisting of mildly reduced graphene oxide modified with silver nanoparticles supported on Co3O4 was synthesized and used for fabricating a nonenzymatic sensor for H2O2. The morphology and composition of the nanocomposite was characterized by transmission electron microscopy, scanning electron microscopy, X-ray powder diffraction and FTIR. The composite was placed on a glassy carbon electrode which then displayed excellent performance in terms of electroreduction of H2O2. The H2O2 sensor, if operated at pH 7.4 at a working potential of 0.4 V (vs. SCE) has the following features: (a) linearity in the 0.1 μM to 7.5 mM concentration range; (b) a sensitivity of 146.5 μA∙mM‾1∙cm‾2; (c) a 35 nM detection limit at a signal-to-noise ratio of 3, and (d) a response time of 2 s. The sensor is long-term stable, well reproducible and selective.

A sandwich structured nanocomposite consisting of mildly reduced graphene oxide modified with silver nanoparticles supported on Co3O4 was synthesized and used for fabricating a nonenzymatic sensor for H2O2.






Similar content being viewed by others
References
Wolfbeis OS, Dürkop A, Wu M, Lin Z (2002) A europium–ion–based luminescent sensing probe for hydrogen peroxide. Angew Chem Int Ed 41:4495–4498
Klassen NV, Marchington D, Mcgowan HCE (1994) H2O2 determination by the is-method and by KMnO4 titration. Anal Chem 292:2921–2925
Albers AE, Okreglak VS, Chang CJ (2006) A FRET-based approach to ratiometric fluorescence detection of hydrogen peroxide. J Am Chem Soc 128:9640–9641
He SH, Shi WB, Zhang XD, Li JA, Huang YM (2010) ß–cyclodextrins–based inclusion complexes of CoFe2O4 magnetic nanoparticles as catalyst for the luminol chemiluminescence system and their applications in hydrogen peroxide detection. Talanta 82:377–383
Chen XM, Wu GH, Cai ZX, Oyama M, Chen X (2014) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181:689–705
Cui K, Song Y, Yao Y, Huang Z, Wang L (2008) A novel hydrogen peroxide sensor based on Ag nanoparticles electrodeposited on DNA-networks modified glassy carbon electrode. Electrochem Commun 10:663–667
Noor AM, Shahid MM, Rameshkumar P, Huang NM (2016) A glassy carbon electrode modified with graphene oxide and silver nanoparticles for amperometric determination of hydrogen peroxide. Microchim Acta 183:911–916
Shi LB, Niu XH, Liu TT, Zhao HL, Lan MB (2015) Electrocatalytic sensing of hydrogen peroxide using a screen printed carbon electrode modified with nitrogen-doped graphene nanoribbons. Microchim Acta 182:2485–2493
Yang Y, Fu RZ, Yuan JJ, Wu SY, Zhang JJ, Wang HY (2008) Highly sensitive hydrogen peroxide sensor based on a glassy carbon electrode modified with platinum nanoparticles on carbon nanofiber heterostructures. Microchim Acta 182:1–9
Wang Q, Zheng JB (2010) Electrodeposition of silver nanoparticles on a zinc oxide film: improvement of amperometric sensing sensitivity and stability for hydrogen peroxide determination. Microchim Acta 169:361–365
Yang XJ, Bai J, Wang YH, Jiang X, He XY (2012) Hydrogen peroxide and glucose biosensor based on silver nanowires synthesized by polyol process. Analyst 137:4362–4367
Liu CY, Hu JM (2009) Hydrogen peroxide biosensor based on the direct electrochemistry of myoglobin immobilized on silver nanoparticles doped carbon nanotubes film. Biosens Bioelectron 24:2149–2154
Wang J, Zhao X, Li J, Kuang X, Fan Y, Wei G, Su Z (2014) Electrostatic assembly of peptide nanofiber-biomimetic silver nanowires onto graphene for electrochemical sensors. ACS Macro Lett 3:529–533
Jiang BB, Wei XW, Wu FH, Wu KL, Chen L, Yuan GZ, Dong C, Ye Y (2014) A non-enzymatic hydrogen peroxide sensor based on a glassy carbon electrode modified with cuprous oxide and nitrogen-doped graphene in a nafion matrix. Microchim Acta 181:1463–1470
Zöpfl A, Sisakthi M, Eroms J, Matysik FM, Strunk C, Hirsch T (2016) Signal enhancement in amperometric peroxide detection by using graphene materials with low number of defects. Microchim Acta 183:83–90
Cui X, Wu SG, Li YX, Wan G (2015) Sensing hydrogen peroxide using a glassy carbon electrode modified with in-situ electrodeposited platinum-gold bimetallic nanoclusters on a graphene surface. Microchim Acta 182:265–272
Li JH, Kuang DZ, Feng YL, Zhang FX, Xu ZF, Liu MQ, Wanga D (2013) Green synthesis of silver nanoparticles–grapheme oxide nanocomposite and its application in electrochemical sensing of tryptophan. Biosens Bioelectron 42:198–206
Han Y, Zheng JJ, Dong SY (2013) A novel nonenzymatic hydrogen peroxide sensor based on Ag–MnO2–MWCNTs nanocomposites. Electrochim Acta 90:35–43
Lin CY, Lai YH, Balamurugan A, Vittal R, Lin CW, Ho KC (2010) Electrode modified with a composite film of ZnO nanorods and Ag nanoparticles as a sensor for hydrogen peroxide. Talanta 82:340–347
Wang LS, Deng JC, Yang F, Chen T (2008) Preparation and catalytic properties of Ag/CuO nano-composites via a new method. Mater Chem Phys 108:165–169
Lu WB, Luo YL, Chang GH, Sun XP (2011) Synthesis of functional SiO2-coated graphene oxide nanosheets decorated with Ag nanoparticles for H2O2 and glucose detection. Biosens Bioelectron 26:4791–4797
Ensafi AA, Asl MJ, Rezaei B (2013) A novel enzyme-free amperometric sensor for hydrogen peroxide based on nafion/exfoliated graphene oxide–Co3O4 nanocomposite. Talanta 103:322–329
Li SJ, Du JM, Zhang JP, Zhang MJ, Chen J (2014) A glassy carbon electrode modified with a film composed of cobalt oxide nanoparticles and graphene for electrochemical sensing of H2O2. Microchim Acta 181:631–638
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Ren W, Fang YX, Wang E, Binary A (2011) Functional substrate for enrichment and ultrasensitive SERS spectroscopic detection of folic acid using graphene oxide/Ag nanoparticle hybrids. ACS Nano 5:6425–6433
Acknowledgments
The authors gratefully acknowledge the financial support of this project by the National Science Fund of China (Nos. 21275116, 21575113), the Specialized Research Fund for the Doctoral Program of Higher Education (Nos. 20126101120023), the Natural Science Fund of Shaanxi Province in China (No. 2013KJXX-25), and the Scientific Research Foundation of Shaanxi Provincial Key Laboratory (Nos. 13JS097, 14JS094, and 15JS100).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 671 kb)
Rights and permissions
About this article
Cite this article
Wu, Q., Sheng, Q. & Zheng, J. Nonenzymatic amperometric sensing of hydrogen peroxide using a glassy carbon electrode modified with a sandwich-structured nanocomposite consisting of silver nanoparticles, Co3O4 and reduced graphene oxide. Microchim Acta 183, 1943–1951 (2016). https://doi.org/10.1007/s00604-016-1829-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1829-0