Abstract
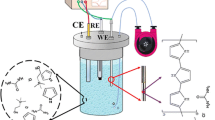
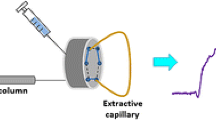
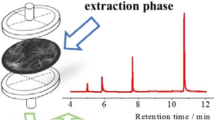
The authors describe an efficient method for microextraction and preconcentration of trace quantities of cationic nitrogen compounds, specifically of anilines. It relies on a combination of electrochemically controlled solid-phase microextraction and on-line in-tube solid-phase microextraction (SPME) using polypyrrole-coated capillaries. Nanostructured polypyrrole was electrically deposited on the inner surface of a stainless steel tube and used as the extraction phase. It also acts as a polypyrrole electrode that was used as a cation exchanger, and a platinum electrode that was used as the anode. The solution to be extracted is passed over the inner surface of the polypyrrole electrode, upon which cations are extracted by applying a negative potential under flow conditions. This method represents an ideal technique for SPME of protonated anilines because it is fast, easily automated, solvent-free, and inexpensive. Under optimal conditions, the limits of detection are in the 0.10–0.30 μg L‾1 range. The method works in the 0.10 to 300 μg L‾1 concentration range. The inter- and intra-assay precisions (RSD%; for n = 3) range from 5.1 to 7.5 % and from 4.7 to 6.0 % at the concentration levels of 2, 10 and 20 μg L‾1, respectively. The EC-in-tube SPME method was successfully applied to the analysis of methyl-, 4-chloro-, 3-chloro and 3,4-dichloroanilines in (spiked) water samples.

ᅟ



Similar content being viewed by others
References
Yang J, Liu HC (2000) IR chemical sensor for detection of chlorinated anilines in aqueous solutions based on ATR waveguides coated with derivatized polystyrene. Analyst 125:1605–1610. doi:10.1039/B004315J
Voyksner RD, Straub R, Keever JT, Freeman HS, Hsu WW (1993) Determination of aromatic amines originating from azo dyes from chemical reduction combined with liquid chromatography/mass spectrometry. Environ Sci Technol 27:1665–1672. doi:10.1021/es00045a025
Dalene M, Skarping G (1985) Trace analysis of amines and isocyanates using glass capillary gas chromatography and selective detection IV. Determination of free aromatic amines using nitrogen-selective detection. J Chromatogr A 331:321–330. doi:10.1016/0021-9673(85)80038-8
Zhao L, Zhu L, Lee HK (2002) Analysis of aromatic amines in water samples by liquid–liquid–liquid microextraction with hollow fibers and high-performance liquid chromatography. J Chromatogr A 963:239–248. doi:10.1016/S0021-9673(02)00544-7
Chiang JS, Huang SD (2008) Simultaneous derivatization and extraction of anilines in waste water with dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometric detection. Talanta 75:70–75. doi:10.1016/j.talanta.2007.10.036
Lewin U, Efer J, Engewald W (1996) High-performance liquid chromatographic analysis with electrochemical detection for residues of explosives in water samples around a former ammunition plant. J Chromatogr A 730:161–167. doi:10.1016/0021-9673(95)01077-7
Adeyoju O, Iwuoha EI, Smyth MR, Leech A (1996) High-performance liquid chromatographic determination of phenols using a tyrosinase-based amperometric biosensor detection system. Analyst 121:1885–1889. doi:10.1039/AN9962101885
Liu XJ, Chen XW, Yang S, Wang XD (2007) Continuous-flow microextration coupled with HPLC for the determination of 4-chloroaniline in Chlamydomonas reinhardtii. Bull Environ Contam Toxicol 78:368–372. doi:10.1007/s00128-007-9200-0
EPA Method 1625, Fed. Reg. US Government Print Office, Washington, DC, 1994
EPA Method 8270B, Fed. Reg. US Government Print Office, Washington, DC, 1994
Sarfaraz-Yazdi A, Es’hagi Z (2006) Comparison of hollow fiber and single-drop liquid-phase microextraction techniques for HPLC determination of aniline derivatives in water. Chromatographia 63:563–569. doi:10.1365/s10337-006-0801-2
Wang X, Fu L, Wei G, Hu J, Zhao X, Liu X, Li Y (2008) Determination of four aromatic amines in water samples using dispersive liquid–liquid microextraction combined with HPLC. J Sep Sci 31:2932–2938. doi:10.1002/jssc.200800273
Börnick H, Grischek T, Worch E (2001) Determination of aromatic amines in surface waters and comparison of their behavior in HPLC and on sediment columns. J Anal Chem 371:607–613. doi:10.1007/s002160101011
Diao CP, Wei CH (2012) Rapid determination of anilines in water samples by dispersive liquid–liquid microextraction based on solidification of floating organic drop prior to gas chromatography–mass spectrometry. Anal Bioanal Chem 403:877–884. doi:10.1007/s00216-012-5907-9
Bhaskar M, Aruna P, Jeevan RJG, Radhakrishnan G (2004) β-Cyclodextrin-polyurethane polymer as solid phase extraction material for the analysis of carcinogenic aromatic amines. Anal Chim Acta 509:39–45. doi:10.1016/j.aca.2003.12.015
Patsias J, Papadopoulou-Mourkidou E (2000) Development of an automated on-line solid-phase extraction–high-performance liquid chromatographic method for the analysis of aniline, phenol, caffeine and various selected substituted aniline and phenol compounds in aqueous matrices. J Chromatogr A 904:171–188. doi:10.1016/S0021-9673(00)00927-4
Brede C, Skjevrak I, Herikstad H (2003) Determination of primary aromatic amines in water food simulant using solid-phase analytical derivatization followed by gas chromatography coupled with mass spectrometry. J Chromatogr A 983:35–42. doi:10.1016/S0021-9673(02)01652-7
Figueiredo EC, Sparrapan R, Sanvido GB, Santos MG, Arruda MAZ, Eberlin MN (2011) Quantitation of drugs via molecularly imprinted polymer solid phase extraction and electrospray ionization mass spectrometry: benzodiazepines in human plasma. Analyst 136:3753–3757. doi:10.1039/C1AN15198C
Vonderheide AP, Montes-Bayon M, Caruso JA (2002) Solid-phase microextraction as a sample preparation strategy for the analysis of seleno amino acids by gas chromatography-inductively coupled plasma mass spectrometry. Analyst 127:49–53. doi:10.1039/B107781C
Yuan B, Li F, Xu D, Fu ML (2013) Comparison of two methods for the determination of geosmin and 2-methylisoborneol in algae samples by stable isotope dilution assay through purge-and-trap or headspace solid-phase microextraction combined with GC/MS. Anal Methods 5:1739–1746. doi:10.1039/C3AY26626E
Mitani K, Kataoka H (2006) Determination of fluoroquinolones in environmental waters by in-tube solid-phase microextraction coupled with liquid chromatography–tandem mass spectrometry. Anal Chim Acta 562:16–22. doi:10.1016/j.aca.2006.01.053
Silva BJG, Lanças FM, Queiroz MC (2008) In-tube solid-phase microextraction coupled to liquid chromatography (in-tube SPME/LC) analysis of nontricyclic antidepressants in human plasma. J Chromatogr B 862:181–188. doi:10.1016/j.jchromb.2007.12.006
Shirakawa H, Louis E, MacDiarmid A, Chiang C, Heeger A (1977) Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x. J Chem Soc Chem Commun 16:578–580. doi:10.1039/C39770000578
Gou Y, Eisert R, Pawliszyn J (2000) Automated in-tube solid-phase microextraction–high-performance liquid chromatography for carbamate pesticide analysis. J Chromatogr A 873:137–147. doi:10.1016/S0021-9673(99)01125-5
Mohammadi A, Yamini Y, Alizadeh N (2005) Dodecylsulfate-doped polypyrrole film prepared by electrochemical fiber coating technique for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons. J Chromatogr A 1063:1–8. doi:10.1016/j.chroma.2004.11.087
Wang YH, Li YQ, Feng JF, Sun C (2008) Polyaniline-based fiber for headspace solid-phase microextraction of substituted benzenes determination in aqueous. Anal Chim Acta 619:202–208. doi:10.1016/j.aca.2008.05.003
Liljegren G, Nyholm L (2003) Electrochemically controlled solid-phase microextraction and preconcentration using polypyrrole coated microarray electrodes in a flow system. Analyst 128:232–236. doi:10.1039/B211398H
Tamer U, Yates B, Galal A, Gbatu T, LaRue R, Schmiesing C, Temsamani K, Ceylan O, Mark HB Jr (2003) Electrochemically aided control of solid phase micro-extraction (EASPME) using conducting polymer-coated solid substrates applicable to neutral analytes. Microchim Acta 143:205–215. doi:10.1007/s00604-003-0056-7
Kalhor H, Alizadeh N (2013) Enhancing sensitivity of ion mobility spectrometry determination of aldehydes by in situ gas phase derivatization with dibutylamine. Int J Ion Mobil Spectrom 16:199–205. doi:10.1007/s12127-013-0119-3
Wu J, Pawliszyn J (2004) Solid-phase microextraction based on polypyrrole films with different counter ions. Anal Chim Acta 520:257–264. doi:10.1016/j.aca.2004.05.019
Wu JC, Pawliszyn J (2001) Preparation and applications of polypyrrole films in solid-phase microextraction. J Chromatogr A 909:37–52. doi:10.1016/S0021-9673(00)01025-6
Eisert R, Pawliszyn J (1997) Automated in-tube solid-phase microextraction coupled to high-performance liquid chromatography. Anal Chem 69:3140–3147. doi:10.1021/ac970319a
Ovais Aziz-Zanjani M, Mehdinia A (2014) A review on procedures for the preparation of coatings for solid phase microextraction. Microchim Acta 181:1169–1190. doi:10.1007/s00604-014-1265-y
Kataoka H (2002) Automated sample preparation using in-tube solid-phase microextraction and its application – a review. Anal Bioanal Chem 373:31–45. doi:10.1007/s00216-002-1269-z
Alhooshani K, Kim TY, Kabir A, Malik A (2005) Sol–gel approach to in situ creation of high pH-resistant surface-bonded organic–inorganic hybrid zirconia coating for capillary microextraction (in-tube SPME). J Chromatogr A 1062:1–14. doi:10.1016/j.chroma.2004.10.103
Acknowledgments
The authors gratefully acknowledge financial support from Tarbiat Modares University
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 429 kb)
Rights and permissions
About this article
Cite this article
Asiabi, H., Yamini, Y., Rezaei, F. et al. Nanostructured polypyrrole for automated and electrochemically controlled in-tube solid-phase microextraction of cationic nitrogen compounds. Microchim Acta 182, 1941–1948 (2015). https://doi.org/10.1007/s00604-015-1534-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1534-4