Abstract
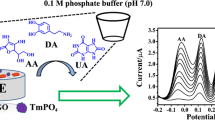
We report on the preparation of a nanocomposite by an electrochemical redox reaction in which graphene oxide is reduced to graphene while nickel-cobalt hexacyanoferrate (III) microparticles are simultaneously formed by oxidation. The microparticles deposit uniformly on the surface of graphene sheets, and the resulting nanocomposite was characterized by scanning electron microscopy, energy dispersive X-ray studies, cyclic voltammetry, and differential pulse voltammetry. Electrochemical studies using a glassy carbon electrode showed the material to possess superior electrocatalytic activity in terms of the oxidation of dopamine (DA) and ascorbic acid (AA). Calibration plots were established for DA in the concentration range from 0.2 to 500 μM (at a potential of 280 mV), and for AA from 0.5 to 200 μM and from 200 to 2,000 μM, (at a potential of 100 mV). These linear ranges are much wider than those of most modified electrodes reported before.

An in-situ process was conducted to prepare NiCo hexacyanoferrate-graphene nanocomposites. Graphene oxide is reduced to graphene while nickel-cobalt hexacyanoferrate (III) microparticles are simultaneously formed by oxidation. The nanocomposites possess superior electrocatalytic activity in terms of the oxidation of dopamine and ascorbic acid.





Similar content being viewed by others
References
Moscone D, DOttavi D, Compagnone D, Palleschi G, Amine A (2001) Construction and analytical characterization of Prussian blue-based carbon paste electrodes and their assembly as oxidase enzyme sensors. Anal Chem 73:2529–2535
Li YX, Li YJ, Hong M, Bin Q, Lin ZY, Lin Z, Cai ZW, Chen GN (2013) Highly sensitive protein molecularly imprinted electro-chemical sensor based on gold microdendrites electrode and Prussian blue mediated amplification. Biosens Bioelectron 42:612–617
Gong HQ, Sun MH, Fan RH, Qian L (2013) One-step preparation of a composite consisting of graphene oxide, Prussian blue and chitosan for electrochemical sensing of hydrogen peroxide. Microchim Acta 180:295–301
Majidi MR, Asadpour-Zeynali K, Hafezi B (2010) Sensing L-cysteine in urine using a pencil graphite electrode modified with a copper hexacyanoferrate nanostructure. Microchim Acta 169:283–288
Fang B, Feng YH, Wang GF, Zhang CH, Gu AX (2011) A uric acid sensor based on electrodeposition of nickel hexacyanoferrate nanoparticles on an electrode modified with multi-walled carbon nanotubes. Microchim Acta 173:27–32
Zhang N, Wang GF, Gu AX, Feng YH, Fang B (2010) Fabrication of Prussian blue/multi-walled carbon nanotubes modified electrode for electrochemical sensing of hydroxylamine. Microchim Acta 168:129–134
Kulesza PJ, Malik MA, Denca A, Strojek J (1996) In situ FT-IR/ATR spectroelectrochemistry of Prussian blue in the solid state. Anal Chem 68:2442–2446
Tacconi NR, Rajeshwar K (2003) Metal hexacyanoferrates: electrosynthesis, in situ characterization and applications. Chem Mater 15:3046–3062
Baioni AP, Vidotti M, Fiorito PA, Ponzio EA, Torresi SIC (2007) Synthesis and characterization of copper hexacyanoferrate nanoparticles for building up long-term stability electrochromic electrodes. Langmuir 23:6796–6800
Mortimer RJ (1997) Electrochromic materials. Chem Soc Rev 26:147–156
Doroftei F, Pinteala T, Arvinte A (2014) Enhanced stability of a Prussian blue/sol–gel composite for electrochemical determination of hydrogen peroxide. Microchim Acta 181:111–120
Fang B, Shen RX, Zhang W, Wang GF, Zhang CH (2009) Electrocatalytic oxidation of hydrazine at a chromium hexacyanoferrate/single-walled carbon nanotube modified glassy carbon electrode. Microchim Acta 165:231–236
Kulesza PJ, Malik MA, Berrettoni M, Giorgetti M, Zamponi S, Schmidt R, Marassi R (1998) Electrochemical charging, countercation accommodation, and spectrochemical identity of microcrystalline solid cobalt hexacyanoferrate. J Phys Chem B 102:1870–1876
Kulesza PJ, MaliM A, Skorek J, Miecznikowski K, Zamponi S, Berrettoni M, Giorgetti M, Marassi R (1999) Hybrid metal cyanometallates electrochemical charging and spectrochemical identity of heteronuclear nickel/cobalt hexacyanoferrate. J Electrochem Soc 146:3757–3761
Kulesza PJ, Malik MA, Schmidt R, Smolinska A, Miecznikowski K, Zamponi S, Czerwinski A, Berrettoni M, Marassi R (2000) Electrochemical preparation and characterization of electrodes modified with mixed hexacyanoferrates of nickel and palladium. J Electroanal Chem 487:57–65
Reddy SJ, Dostal A, Scholz F (1996) Solid state electrochemical studies of mixed nickel-iron hexacyanoferrates with the help of abrasive stripping voltammetry. J Electroanal Chem 403:209–212
Cui XP, Hong L, Lin XQ (2002) Electrochemical preparation, characterization and application of electrodes modified with hybrid hexacyanoferrates of copper and cobalt. J Electroanal Chem 526:115–124
Shi LH, Wu T, He P, Li D, Sun CY, Li JH (2005) Amperometric sensor for hydroxylamine based on hybrid nickel-cobalt hexacyanoferrate modified electrode. Electroanalysis 17:2190–2194
Yavari F, Koratkar N (2012) Graphene-based chemical sensors. J Phys Chem Lett 3:1746–1753
Palanisamy S, Ku SH, Chen SM (2013) Dopamine sensor based on a glassy carbon electrode modified with a reduced graphene oxide and palladium nanoparticles composite. Microchim Acta 180:1037–1042
Zhang HF, Shuang SM, Sun LL, Chen AJ, Qin Y, Dong C (2014) Label-free aptasensor for thrombin using a glassy carbon electrode modified with a graphene-porphyrin composite. Microchim Acta 181:189–196
Sun CL, Chang CT, Lee HH, Zhou JG, Sham TK, Pong WF (2011) Microwave-assisted synthesis of a core-shell MWCNT/GONR heterostructure for the electrochemical detection of ascorbic acid, dopamine and uric acid. ACS Nano 5:7788–7795
Salimi A, Abdi K, Khayatian GR (2004) Amperometric detection of dopamine in the presence of ascorbic acid using a nafion coated glassy carbon electrode modified with catechin hydrate as a natural antioxidant. Microchim Acta 144:161–169
Zheng YJ, Huang ZJ, Zhao CF, Weng SH, Zheng W, Lin XH (2013) A gold electrode with a flower-like gold nanostructure for simultaneous determination of dopamine and ascorbic acid. Microchim Acta 180:537–544
Li HX, Gao Q, Chen LS, Hao WL (2012) Photocurrent determination ascorbic acid using an n-silicon electrode modified by platinum and cobalt hexacyanoferrate films. Sens Actuators B 173:540–546
Mashhadizadeh MH, Yousefi T, Golikand AN (2012) A nickel hexacyanoferrate and poly (1-naphthol) hybrid film modified electrode used in the selective electroanalysis of dopamine. Electrochim Acta 59:321–328
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Safavi A, Kazemi SH, Kazemi H (2011) Electrochemically deposited hybrid nickel–cobalt hexacyanoferrate nanostructures for electrochemical supercapacitors. Electrochim Acta 56:9191–9196
Ferrari AC (2007) Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Comm 143:47–57
Liu Y, Huang Y, Xie Y, Yang Z, Huang H, Zhou Q (2012) Preparation of highly dispersed CuPt nanoparticles on ionic-liquid-assisted graphene sheets for direct methanol fuel cell. Chem Eng J 197:80–87
Li SJ, Deng DH, Shi Q, Liu SR (2012) Electrochemical synthesis of a graphene sheet and gold nanoparticle-based nanocomposite, and its application to amperometric sensing of dopamine. Microchim Acta 177:325–331
Zhang F, Li Y, Gu Y, Wang Z, Wang C (2011) One-pot solvother-mal synthesis of a Cu2O/Graphene nanocomposite and its application in an electrochemical sensor for dopamine. Microchim Acta 173:103–109
Shankaran DR, Narayanan SS (2001) Amperometric sensor for hydrazine determination based on mechanically immobilized nickel hexacyanoferrate modified electrode. Russ J Electrochem 37:1322–1326
He P, Wang W, Du LC, Dong FQ, Deng YQ, Zhang TH (2012) Zeolite a functionalized with copper nanoparticles and graphene oxide for simultaneous electrochemical determination of dopamine and ascorbic acid. Anal Chim Acta 739:25–30
Yan W, Yan X (2012) Glassy carbon electrode modified with poly (dibromofluorescein) for the selective determination of dopamine and uric acid in the presence of ascorbic acid. Microchim Acta 178:123–130
Ensafi AA, Taei M, Khayamian T, Arabzadeh A (2010) Highly selective determination of ascorbic acid, dopamine, and uric acid by differential pulse voltammetry using poly (sulfonazo III) modified glassy carbon electrode. Sens Actuators B 147:213–221
Jin GD, Chen D, Hu XY, Zhang R (2009) Simultaneous electrochemical determination of dopamine, ascorbic acid and uric acid using poly (acid chrome blue K) modified glassy carbon electrode. Sens Actuators B 138:174–181
Zhu SY, Li HJ, Niu WX, Xu GB (2009) Simultaneous electrochemical determination of uric acid, dopamine, and ascorbic acid at single-walled carbon nanohorn modified glassy carbon electrode. Biosens Bioelectron 25:940–943
Sun YX, Sheng FW (2006) Simultaneous determination of dopamine and ascorbic acid at a triazole self-assembled monolayer-modified gold electrode. Microchim Acta 154:115–121
Wang GF, Sun JG, Zhang W, Jiao HF, Fang B (2009) Simultaneous determination of dopamine, uric acid and ascorbic acid with LaFeO3 nanoparticles modified electrode. Microchim Acta 164:357–362
Acknowledgments
This work was supported by the Basic Science Research Fund in Xidian University (No. JB141404).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 109 kb)
Rights and permissions
About this article
Cite this article
Wang, Q., Tang, Q. Improved sensing of dopamine and ascorbic acid using a glassy carbon electrode modified with electrochemically synthesized nickel-cobalt hexacyanoferrate microparticles deposited on graphene. Microchim Acta 182, 671–677 (2015). https://doi.org/10.1007/s00604-014-1371-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1371-x