Abstract
Aims
Type 2 diabetes mellitus (T2DM) is a progressive disease, often requiring exogenous insulin therapy and treatment intensification. Despite new therapies, most patients do not reach the recommended HbA1c targets, among them a significant proportion of patients on premixed insulins. The aim was to summarize published data in Adriatic countries on effectiveness of insulin glargine based therapy in type 2 diabetic patients suboptimally controlled on premix insulin.
Methods
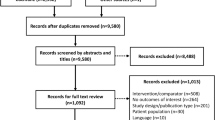
A meta-analysis was carried out in major medical databases up to April 2014, focusing on Adriatic region. We searched observational studies with duration of at least 6 months, evaluating effectiveness and safety of insulin glargine (IGlar), in combination with OAD or bolus insulin in patients with T2 failing premixed insulin therapy. Outcomes included values of HbA1c, fasting blood glucose and two hours post-prandial glucose concentration as well as changes in body mass index after at least 6 months of study duration.
Results
Three prospective, observational, multicentric trials (698 patients in total) were included. The basal bolus regimen with glargine significantly reduced HbA1c (Mean Difference, MD=2.27, CI [1.76, 2.78]), fasting glucose (MD=5.15, CI [4.86, 5.44]) and 2-hours postprandial glucose concentration (MD=6.94, CI [6.53, 7.34]). No significant changes were found in BMI after switching from premixes to IGlar based treatment.
Conclusion
Insulin glargine based therapy following premix failure is efficacious and safe option of type 2 diabetes treatment intensification.




Similar content being viewed by others
References
Chen L, Magliano DJ, Zimmet PZ (2012) The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 8:228–236
Chan JC et al (2009) Diabetes Care 32:227–233
Monami M, Ragghianti B, Zannoni S, Vitale V, Nreu B, Mannucci E (2016) Identification of predictors of response to basal insulin and DPP4 inhibitors in patients with type 2 diabetes failing to other therapies. Acta Diabetol 53:35–40
Lavernia F (2011) What options are available when considering starting insulin: premix or basal? Diabetes Technol Ther 13(Suppl 1):S85–S92
Tibaldi JM (2011) Intensifying insulin therapy in type 2 diabetes mellitus: dosing options for insulin analogue premixes. Clin Ther 33(11):1630–1642
Korytkowski M (2002) When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord 26:S18–S24
Bellia A, Babini AC, Marchetto PE, Arsenio L, Lauro D, Lauro R (2014) Effects of switching from NPH insulin to insulin glargine in patients with type 2 diabetes: the retrospective, observational LAUREL study in Italy. Acta Diabetol 51:269–275
Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S (2008) Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care 31:20–25
Roach P, Trautmann M, Arora V, Sun B, Anderson J (1999) Improved postprandial blood glucose control and reduced nocturnal hypoglycaemia during treatment with two novel insulin lispro-protamine formulations, insulin lispro Mix25 and Insulin Lispro Mix50. Clin Ther 21:523–534
Koivisto VA, Tuominen JA, Ebeling P (1999) Lispro Mix25 insulin as premeal therapy in type 2 diabetic patients. Diabetes Care 22:459–462
Garber et al (2006) 2006 Premixed Insulin Analogues for the Treatment of Diabetes Mellitus. Drugs 66(1):31–49
Holman RR, Farmer AJ, Davies MJ et al (2009) Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl Med 361:1736–1747
Schiel R, Schweitzer MA, Muller UA (2003) Quality of glycemic control and well-being of patients with type 2 diabetes mellitus after switching from conventional insulin therapy to once-daily insulin glargine in combination with oral anti-diabetic drugs—SWITCH pilot, a prospective randomized trial. Diabetologia 46:A272 (Abstract788)
Hammer H, Schneider K, Niemoller E, Schweitzer MA (2005) Patients with type 2 diabetes inadequately controlled on premixed insulin: effect of initiating insulin glargine plus oral agents on glycemic control in daily practice. Diabetes 54:518 (Abstract 2153)
Zjacic V et al (2012) Efficacy and safety of a basal-bolus regimen with insulin glargine in patients with type 2 diabetes after failing pre-mix insulin therapy: a multicentre postmarketing study. Diabetol Croat 41(1):41-48
Buturovic et al (2013) Improved glycemic control with insulin glargine as part of basal bolus in regimen T2DM inadequately controlled on premixed therapy. Med Arh 67(5):342–345
Pemovska et al (2014) Mac. Evaluation of the effectivness and safety of basal based therapy with insulin glargine and prandial inaulin in patients with Type 2 diabetes poorly controlled with premixed insulins. Med Rev 68(1):44–48
Pešić M, Živić S, Radenković S, Velojić M, Dimić D, Antić S (2007) Comparison between basal insulin glargine and NPH insulin in patients with diabetes type 1 on conventional intensive insulin therapy. Vojnosanit Pregl 64(4):247–252
Damnjanović I, Veličković-Radovanović R, Kocić R, Zlatković-Guberinić S, Sokolović D, Đinđić N, Conić I (2011) Influence of beta-blockers on insulin resistance in patients with diabetes mellitus type 2. Acta Med Median 50(4):23–28
Damnjanovic I, Velickovic-Radovanovic R, Catic-Djordjevic A, Kocic R, Ciric V, Conic I, Bjelakovic LJ (2008) Pharmacokinetic and pharmacodynamic modelling of base insulins and analogs. Acta Med Median 47(4):15–19
Davies M, Sinnassamy P, Fred Storms F et al (2008) Insulin glargine-based therapy improves glycemic control in patients with type 2 diabetes sub-optimally controlled on premixed insulin therapies. Diabetes Res and Clin Pract 79:368–375
Fritsche A, Larbig M, Owens D, Haring HU (2010) Comparison between a basal-bolus and a premixed insulin regimen in individuals with type 2 diabetes—results of the GINGER study. Diabetes Obes Metab 12:115–123
Qayyum R, Bolen S, Maruthur N et al (2008) Systematic review: comparative effectiveness and safety of premixed insulin analogues in type 2 diabetes. Ann Intern Med 149:549–559
American Diabetes Association (2013) Standards of medical care in diabetes-2014. Diabetes Care 37:S14–S80. doi:10.2337/dc14-S014
International Diabetes Federation (2012) Clinical guidelines task force. Global guideline for type 2 diabetes. http://www.idf.org/global-guideline-type-2-diabetes-2012
Review Manager (RevMan) [Computer program] (2012) Version 5.2. The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen
Acknowledgments
We would like to thank Radenka Munjas Samarin for assistance with graphic design and editorial support, funded by Sanofi in the preparation of this manuscript. This study was sponsored by Sanofi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Maja Cigrovski Berković declares associations (lecturer, member of advisory board, clinical trial investigator) with the following companies: Astra-Zeneca, Boehringer-Ingelheim, Eli Lilly, Merck Sharpe & Dohme, Novartis, NovoNordisk, and Sanofi-Aventis. Goran Petrovski declares associations (lecturer, clinical trial investigator) with the following companies: Eli Lilly, NovoNordisk, Sanofi-Aventis, Medtronic and Alkaloid. Natasa Grulovic is an employee of Sanofi-Aventis.
Ethical standard
In the Manuscript we presented and analyzed data from different studies as a meta-analysis. All presented data comes from studies that were approved by local Ethical Committees.
Human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was obtained from all patients for being included in the studies analyzed in the present meta-analysis.
Additional information
Managed by Antonio Secchi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cigrovski Berkovic, M., Petrovski, G. & Grulovic, N. Effectiveness of insulin glargine in type 2 diabetes mellitus patients failing glycaemic control with premixed insulin: Adriatic countries data meta-analysis. Acta Diabetol 53, 709–715 (2016). https://doi.org/10.1007/s00592-016-0861-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-016-0861-1