Abstract
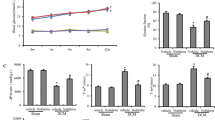
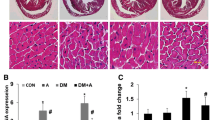
Over-activation of the local chymase–angiotensin II (Ang II) system has a dominant role in diabetic cardiomyopathy. Astragalus polysaccharides (APS) are used in traditional Chinese medicine to boost immunity. In this study, we investigated the effects of APS treatment on cardiac function, myocardial collagen expression, cardiac ultrastructure, cardiac matrix metalloproteinase (MMP) activity, levels of plasma glycosylated serum protein (GSP), and myocardial enzymes, and the expression of Ang II, chymase, and angiotensin-converting enzyme (ACE) in the diabetic hamster myocardium. Diabetes was induced by a single injection of streptozotocin (60 mg/kg ip). The experimental groups consisted of normal control (n = 15), diabetic (n = 15), insulin-treated diabetic (n = 15, NPH 1–2 U/day ip), and APS-treated diabetic (n = 30, APS 1–2 g/kg/day orally for 10 weeks) hamsters. Diabetic hamsters treated with insulin or APS exhibited significantly decreased blood glucose, plasma GSP, and myocardial enzymes, as well as improvements in cardiac function and cardiac ultrastructure. Compared with insulin treatment, APS treatment significantly reduced myocardial collagen (type I and III) expression and lowered cardiac MMP-2 activity, myocardial Ang II levels, myocardial chymase expression, and p-ERK1/2 kinase expression. In diabetic hamsters, myocardial ACE expression and plasma Ang II levels was not altered by insulin or APS treatment. These results indicate that treatment of diabetic hamsters with APS inhibited the local chymase–Ang II system and improved markers of diabetic cardiomyopathy.






Similar content being viewed by others
References
Flack JM, Hamaty M, Staffilen BA (2004) Renin-angiotensin-aldosterone-kinin system influences on diabetic vascular disease and cardiomyopathy. Miner-Electrolyte Metab 24(6):412–422
Urata H, Kinoshita A, Misono KS (2000) Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem 265(36):22348–22359
Nishimoto M, Takai S, Sawada Y (2001) Chymase-dependent angiotensin II formation in the saphenous vein versus the internal thoracic artery. J Thorac Cardiovasc Surg 121(4):729–736
Ni Y, Su Q, Liu X (1998) Experimental study of optimized techniques of water decoction extraction of Astragalus polysaccharide. China J Chin Med 23(6):284–286
Chen W, Yu M-H (2004) Effects of astragalus polysaccharide on gene expression profiles in islets of NOD mice with microarray technique. Chin J Endocrinol Metab 20(6):545–548
Chen W, Yu M-H, Li Y-M (2007) Effects of astragalus polysaccharides on ultrastructure and oxidation/apoptosis related cytokines’ gene expression of NOD mice’s islets. Fudan Univ J Med Sci 34(2):269–272
Chen W, Xia Y-P, Yu M-H (2007) Astragalus polysaccharides effect on pancreatic histopathology. China J Modern Med 17(2):146–148
Chen W, Li Y-M, Yu M-H (2007) Immunoregulation effects of astragalus polysaccharides on T helper lymphocyte subgroups in nonobese diabetic mice. China J Modern Med 17(1):28–31
Cong L, Li Y-M, Yu M-H (2006) Effect of astragalus polysaccharides on the expression of myocardial collagen in hamsters with diabetic cardiomyopathy. Chin J Clin Rehab 10(7):64–66
Sunni S, Bishop SP, Kent SP (2001) Diabetic cardiomyopathy, a morphological study of intramyocardial arteries. Arch Pathol Lab Med 110(5):375–389
Takai S, Sakaguchi M, Jin DL (2001) Different angiotensin II-forming pathways in human and rat vascular tissues. Clin Chim Acta 305(1–2):191–203
Packer M, Poole-Wilson PA, Armstrong PW (1999) Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation 100(23):2312–2322
Uchiyama-Tanaka Y, Matsubara H, Nozawa Y (2001) Angiotensin II signaling and HB-EGF shedding via metalloproteinase in glomerular mesangial cells. Kidney Int 60(6):2153–2161
Sekine S, Nitta K, Uchida K (2003) Possible involvement of mitogen-activated protein kinase in the angiotensin II-induced fibronectin synthesis in renal interstitial fibroblasts. Arch Biochem Biophys 415(1):63–77
Awazu M, Ishikura K, Hida ML (1999) Mechanisms of mitogen-activated protein kinase activation in experimental diabetes. J Am Soc Nephrol 10(4):738–744
Takai S, Miyazaki M (2002) The role of chymase in vascular proliferation. Drug News Perspect 15(5):278–285
Jin D, Takai S, Yamada M (2000) The functional ratio of chymase and angiotensin converting enzyme in angiotensin I-induced vascular contraction in monkeys, dogs and rats. Jpn J Pharmacol 84(4):449–455
Jin D, Takai S, Yamada M (2001) Possible roles of cardiac chymase after myocardial infarction in hamster hearts. Jpn J Pharmacol 86(2):203–208
Miyazaki M, Takai S (2001) Local angiotensin II-generating system in vascular tissues: the roles of chymase. Hypertens Res 24(3):189–194
Yu CM, Tipoe GL, Wing-Hon Lai K (2001) Effects of combination of angiotensin converting enzyme inhibitor and angiotensin receptor antagonist on inflammatory cellular infiltration and myocardial interstitial fibrosis after acute myocardial infarction. J Am Coll Cardiol 38(4):1207–1211
Frank BT, Rossall JC, Caughey GH (2001) Mast cell tissue inhibitor of metalloproteinase-1 is cleaved and inactivated extracellularly by alpha-chymase. J Immunol 166(4):2783–2793
Browe GL, Chancey AL, Thanigaraj S (2002) Cause and effect relationship between myocardial mast cell number and matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol 283(2):H518–H523
de Almeida A, Mustin D, Forman MF (2002) Effects of mast cells on the behavior of isolated heart fibroblasts: modulation of collagen remodeling and gene expression. J Cell Physiol 191(1):51–62
Hara M, Ono K, Hwang MW (2002) Evidence for a role of mast cells in the evolution to congestive heart failure. J Exp Med 195(3):375–381
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W., Yu, MH., Li, YM. et al. Beneficial effects of astragalus polysaccharides treatment on cardiac chymase activities and cardiomyopathy in diabetic hamsters. Acta Diabetol 47 (Suppl 1), 35–46 (2010). https://doi.org/10.1007/s00592-009-0116-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-009-0116-5