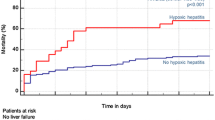
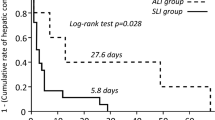
Abstract
Background
Corticosteroid therapy has been commonly administered to patients with acute liver injury (ALI)/acute liver failure (ALF) in Japan to prevent the development of hepatic encephalopathy, but the appropriate timing to start corticosteroid therapy has not been determined and optimal response evaluation of the therapy has not been conducted. We prospectively investigated the optimal timing to start therapy on the established severity indication: the Japan Hepatic Encephalopathy Prediction Model (JHEPM) and prothrombin time (PT).
Methods
This prospective observational study enrolled 469 patients with ALI/ALF from 2004 to 2015. We evaluated 44 patients with ALF on high-dose corticosteroid therapy before hepatic coma development. The predictive performance for coma development was assessed using the receiver operator curve method in both PT and JHEPM probability the day before administering high-dose corticosteroid therapy.
Results
Among these patients, nine developed hepatic coma after the therapy. Selection bias was adjusted using propensity score method. High-dose corticosteroid therapy tended to decrease the risk of coma development although there was no statistical significance. The cut-off value of 53%, 1.95, and 39% in JHEPM probability, PT-international normalized ratio (PT-INR), and PT activity, respectively, showed high sensitivity and specificity.
Conclusions
We propose the appropriate timing to start high-dose corticosteroid therapy in patients with ALI/ALF; 40% of JHEPM probability, 1.53 of PT-INR, and 52% of PT because these values were theoretically discriminated at 98% coverage to the patients with coma. Because the study contained selection bias, the appropriate timing for therapy should be confirmed in a future prospective study.





Similar content being viewed by others
Abbreviations
- ALF:
-
Acute liver failure
- ALI:
-
Acute liver injury
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate transaminase
- AUROC:
-
Area under the receiver operating characteristic curve
- JHEPM:
-
Japan Hepatic Encephalopathy Prediction Model
- Cre:
-
Creatinine
- PT:
-
Prothrombin time activity
- PT-INR:
-
Prothrombin time-international normalized ratio
- ROC:
-
Receiver–operator curve
- TBil:
-
Total bilirubin
- WBC:
-
White blood cell counts
References
Sugawara K, Nakayama N, Mochida S. Acute liver failure in Japan: definition, classification, and prediction of the outcome. J Gastroenterol. 2012;47:849–61.
Oketani M, Ido A, Tsubouchi H. Changing etiologies and outcomes of acute liver failure: a perspective from Japan. J Gastroenterol Hepatol. 2011;26(Suppl 1):65–71.
Yamashiki N, Sugawara Y, Tamura S, et al. Outcomes after living donor liver transplantation for acute liver failure in Japan: results of a nationwide survey. Liver Transpl. 2012;18:1069–77.
Ware AJ, Jones RE, Shorey JW, et al. A controlled trial of steroid therapy in massive hepatic necrosis. Am J Gastroenterol. 1974;62:130–3.
Gregory PB, Knauer CM, Kempson RL, et al. Steroid therapy in severe viral hepatitis. A double-blind, randomized trial of methyl-prednisolone versus placebo. N Engl J Med. 1976;294:681–7.
Redeker AG, Schweitzer IL, Yamahiro HS. Letter: Randomization of corticosteroid therapy in fulminant hepatitis. N Engl J Med. 1976;294:728–9.
Fujiwara K, Yasui S, Yonemitsu Y, et al. Efficacy of high-dose corticosteroid in the early stage of viral acute liver failure. Hepatol Res. 2014;44:491–501.
Ohira H, Takahashi A. Current trends in the diagnosis and treatment of autoimmune hepatitis in Japan. Hepatol Res. 2012;42:131–8.
Kakisaka K, Kataoka K, Suzuki Y, et al. Necrotic cell death and suppression of T-cell immunity characterized acute liver failure due to drug-induced liver injury. Cytokine. 2016;86:21–8.
Yasui S, Fujiwara K, Yonemitsu Y, et al. Clinicopathological features of severe and fulminant forms of autoimmune hepatitis. J Gastroenterol. 2011;46:378–90.
Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13.
Karkhanis J, Verna EC, Chang MS, et al. Steroid use in acute liver failure. Hepatology. 2014;59:612–21.
Takikawa Y, Endo R, Suzuki K, et al. Early prediction of short-term development of hepatic encephalopathy in patients with acute liver disease unrelated to paracetamol. A prospective study in Japan. J Hepatol. 2009;51:1021–9.
Kakisaka K, Kataoka K, Onodera M, et al. Alpha-fetoprotein: a biomarker for the recruitment of progenitor cells in the liver in patients with acute liver injury or failure. Hepatol Res. 2014;45:E12–20.
Kakisaka K, Kataoka K, Kuroda H, et al. Predictive formula for acute liver failure is useful for predicting the prognosis of patients with acute-on-chronic liver failure. Hepatol Res. 2016;46:459–67.
Oketani M, Ido A, Nakayama N, et al. Etiology and prognosis of fulminant hepatitis and late-onset hepatic failure in Japan: summary of the annual nationwide survey between 2004 and 2009. Hepatol Res. 2013;43:97–105.
Randomised trial of steroid therapy in acute liver failure. Report from the European Association for the Study of the Liver (EASL). Gut. 1979;20:620–623.
Franchini M, Lippi G. Prothrombin complex concentrates: an update. Blood Transfus. 2010;8:149–54.
Takikawa Y, Harada M, Wang T, et al. Usefulness and accuracy of the international normalized ratio and activity percent of prothrombin time in patients with liver disease. Hepatol Res. 2014;44:92–101.
Kudo M, Todo A, Ikekubo K, et al. Quantitative assessment of hepatocellular function through in vivo radioreceptor imaging with technetium 99m galactosyl human serum albumin. Hepatology. 1993;17:814–9.
Kakisaka K, Kuroda H, Abe T, et al. Hepatic hemodynamics and elevation of liver stiffness as possible predictive markers of late-onset hepatic failure. Intern Med. 2016;55:1091–5.
Acknowledgements
We thank Koko Motodate for providing excellent secretarial support. This study was supported in part by Grants-in-Aid from the Ministry of Health, Labor and Welfare of Japan to the Intractable Hepatobiliary Diseases Study Group (JP16K21307 and JP16K09370).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
535_2017_1306_MOESM2_ESM.pptx
Supplementary material 2 (PPTX 240 kb) Supplemental Figure Legend. Serial changes of PT and PT-INR are compared between patients with high-dose corticosteroid therapy (Pulse) and those without the Pulse. A and B: One hundred twenty-one patients were divided into the following two groups: 77 patients without high-dose corticosteroid therapy and 44 patients with the therapy. Serial changes in PT-INR (A) and PT (B) are presented as line charts. Y axis indicates each value. X axis indicates timepoints of each evaluation. Statistical significance was evaluated using the Friedman test, and defined as a p value < 0.05. No significant difference is presented as n.s
Rights and permissions
About this article
Cite this article
Kakisaka, K., Kataoka, K., Suzuki, Y. et al. Appropriate timing to start and optimal response evaluation of high-dose corticosteroid therapy for patients with acute liver failure. J Gastroenterol 52, 977–985 (2017). https://doi.org/10.1007/s00535-017-1306-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1306-5