Abstract
Hepatocellular carcinoma (HCC) is one of the world’s most aggressive diseases and carries a poor prognosis for patients. Recent evidence suggests that HCC is organized by cancer stem cells (CSCs), which are a subset of cells with stem cell-like features. CSCs are considered a pivotal target for the eradication of cancer, and liver CSCs have been investigated using various stem cell markers. Several hepatic stem/progenitor markers have been shown to be useful for isolating putative CSCs from HCC, although the expression patterns and phenotypic diversity of CSCs purified by these markers remain obscure. Recently, we found that liver CSCs defined by different markers show unique features of tumorigenicity and metastasis, with phenotypes closely associated with committed liver lineages. Furthermore, our data suggest that these distinct CSCs collaborate to orchestrate the tumorigenicity and metastasis of HCC. In this review article, we summarize the recent advances in understanding the pathogenesis and heterogeneity of liver CSCs.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of death from cancer worldwide [1]. Its prevalence is mostly attributed to hepatitis B virus or hepatitis C virus infection, and high incidence is observed in Asia and Africa [2]. Increasing occurrences and mortality from HCC have also been observed in most industrialized countries [3]. Therefore, there is an urgent need to develop effective diagnostic and treatment strategies against this disease.
HCC is a heterogeneous disease in terms of morphology, biological behavior, response to treatment, and molecular profile [4]. This heterogeneity has traditionally been explained by the clonal evolution of tumor cells resulting from the progressive accumulation of multiple genetic and epigenetic changes [5, 6]. However, recent studies suggest that its heterogeneity may result from the hierarchical organization of tumor cells by a subset of cells with stem and progenitor cell features known as cancer stem cells (CSCs) [7]. CSCs are highly tumorigenic, metastatic, chemo- and radiotherapy resistant, responsible for tumor relapse after therapy, and able to divide symmetrically or asymmetrically to orchestrate the tumor mass [8]. Therefore, they are considered to be a pivotal target for eradicating HCC [9]. In this review, we summarize recent findings on liver CSCs in terms of heterogeneity and discuss an HCC treatment strategy that targets them.
CSC hypothesis
Cancer cells and stem cells have similar capabilities with respect to self-renewal, limitless division, and the generation of heterogeneous cell populations. The observation of these similarities many years ago led to the proposal that cancer might be a type of abnormal stem cell disease [10], a concept which has recently been revisited [11]. The generally acknowledged definition of a CSC is a cell within a tumor that possesses the ability to self-renew and to give rise to heterogeneous lineages of cancer cells that comprise tumors in immunodeficient mice [11]. Experimentally, putative CSCs have been isolated using cell surface markers specific for normal stem cells. Stem cell-like features of CSCs have been confirmed by functional in vitro clonogenicity and in vivo tumorigenicity assays. Moreover, accumulating evidence suggests that CSCs play a role in perpetuating various cancers including leukemia and solid tumors [12–18].
In HCC, several markers are reported to enrich the CSC population, including the epithelial cell adhesion molecule (EpCAM), CD133, CD90, CD44, CD24, CD13, and oval cell marker OV6, as well as Hoechst dye efflux or aldehyde dehydrogenase activities [19–25]. Most of these markers are expressed in normal hepatic progenitors known as oncofetal markers [20–22, 26–35]. These marker-positive cells were experimentally confirmed to be more tumorigenic than marker-negative cells in immunodeficient mice using cell lines [9]. Among them, calcium channel α2δ1 isoform5, EpCAM, CD90, and CD133 are the markers confirmed thus far to enrich CSCs from primary HCCs [36, 37]. Recent studies have shown that some of these liver CSC markers are also functionally involved in the maintenance of CSC features (Table 1). EpCAM enhances Wnt signaling in ES cells and cancer [38, 39], and CD133 expression may maintain CD133+ liver CSCs through the activation of neurotensin/IL-8/CXCL1 signaling [40]. CD44 regulates the redox status [41], while CD13 decreases cell damage induced by oxidative stress after exposure to genotoxic reagents [19]. Furthermore, a recent study demonstrated that the calcium channel α2δ1 isoform5, recognized by a monoclonal antibody 1B50-1, is expressed in liver CSCs and regulates calcium influx and ERK signaling [37]. Thus, the functional involvement of most liver CSC markers potentially makes them a good target for the eradication of liver CSCs. In particular, cell surface markers detected in liver CSCs may be good targets for immunotherapy.
Heterogeneity of liver CSCs
As described above, various hepatic progenitor markers have been detected in the population of liver CSCs. Purified cell populations using certain stem cell markers show CSC features such as high tumorigenicity, an invasive nature, and chemo- and radiotherapy resistance. However, it is unclear how these markers are expressed in primary HCC tissues or HCC cell lines. It is also unclear whether the CSCs expressing these markers exist in all HCCs or are restricted to a certain subtype. This is an especially important issue when treating HCC patients using molecularly targeted therapy against certain marker-positive CSCs.
In normal fetal livers, hepatoblasts express the biliary markers CK19 and EpCAM, as well as the hepatocyte markers albumin and alpha fetoprotein (AFP) [26, 27, 42, 43]. In addition, numerous studies have demonstrated that hepatic progenitor cells express a variety of markers putatively detected in various ectodermal or mesodermal lineages, including nestin, NCAM, CD34 and c-Kit, CD133, CD90, E-cadherin, and Dlk1 [44]. Hepatoblasts are also considered a heterogeneous population potentially organized in a hierarchical manner with various degrees of differentiation that may be related to their expression of stem cell markers [45]. Indeed, recent studies demonstrated that the characteristics of hepatic progenitors expressing different markers show distinct natures [32, 46]. Normal EpCAM+ and CD90+ oval cells represent two distinct populations: the former expresses classical oval cell markers such as AFP, OV-1, and CK19, and the latter expresses desmin and a-SMA but not AFP, OV-1, or CK19, which indicates that CD90+ populations are more likely to be mesenchymal cells.
We explored the expression patterns of the representative liver CSC markers CD133, CD90, and EpCAM in primary HCC, and found that EpCAM+ and CD90+ CSCs show different gene expression patterns and cell morphology [36]. We further explored the tumorigenic capacity of sorted cells isolated from 15 primary HCCs and 7 liver cancer cell lines [36]. Although the number of samples analyzed was small, tumorigenic EpCAM+, CD133+, or CD90+ CSCs were obtained in 26.6 % (n = 4), 20 % (n = 3), and 13.3 % (n = 2) of 15 HCCs, respectively, when xenotransplanted into NOD/SCID mice.
Interestingly, no EpCAM/CD90 double positive cells were detected in primary HCC, and EpCAM+ and CD90+ cells were distinctive with different tumorigenic/metastatic capacities; that is, EpCAM+ cells were associated with a high tumorigenic capacity and hepatic epithelial stem cell features, while CD90+ cells had a metastatic propensity with mesenchymal vascular endothelial cell features. Importantly, the existence of EpCAM+ cells correlated with high serum AFP values with a tendency for portal vein invasion, whereas the existence of CD90+ cells was associated with a high incidence of distant organ metastasis. Furthermore, CD90+ CSCs abundantly expressed c-Kit and showed chemosensitivity against the c-Kit inhibitor imatinib mesylate, whereas EpCAM+ CSCs showed no such chemosensitivity. These data demonstrate that liver CSCs are not a single entity but exist heterogeneously with distinct CSC marker expression, suggesting that no common liver CSCs expressing particular stem cell markers exist in all HCCs. Our data also indicate that the presence of distinct CSCs is a key determinant of cancer phenotypes in terms of tumorigenicity and metastatic propensity, which may influence the clinical outcome of HCC.
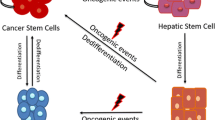
The distinct nature of EpCAM+ and CD90+ liver CSCs raises the question whether these different types of CSCs originate from the same or different type of cells. This question remains elusive, but a recent study investigating three independent cell clones established from the same HCC specimen revealed that these clones maintain common karyotype abnormality but express EpCAM, CD90, and CD133 distinctively with different chemosensitivities against sunitinib [47], suggesting that distinct liver CSCs expressing different markers may originate from the same type of cells. In terms of liver CSC origin, a recent study demonstrated that acquisition of liver CSC properties is independent of the cell of origin, and liver CSCs can originate from hepatic progenitor cells, hepatoblasts, or adult hepatocytes in mice by forced H-Ras/SV40LT induction and subsequent oncogenic reprogramming [48]. In addition, another study has demonstrated the unexpected plasticity of normal mature hepatocytes to dedifferentiate into progenitor cells in rats [49], and this type of plasticity has also been reported in breast non-CSCs [50, 51]. Given the cellular plasticity reported in normal and cancer cells described above, it is reasonable to speculate that a similar plasticity may exist in EpCAM+ and CD90+ CSCs that can convert their tumorigenic/metastatic phenotypes and marker expression status. Further studies are required to clarify the role of cell plasticity on heterogeneity of HCC [36].
Interaction of distinct cell lineages in liver organogenesis and hepatocarcinogenesis
Embryogenesis is characterized by the ordered emergence of an organism made up of a multitude of stem and differentiated cells. Various signaling pathways play crucial roles in the dynamic cell proliferation and motility of organogenesis [52]. For example, in liver organogenesis, liver specification signaling is activated at the ventral endoderm (hepatic endoderm) by the paracrine secretion of fibroblast growth factor (FGF) and bone morphogenic protein (BMP) from the cardiac mesoderm and septum transversum, respectively [53–55]. Wnt/beta-catenin signaling may also induce hepatic specification [56]. Activation of these signaling pathways results in the formation of the liver bud from the hepatic endoderm. The liver bud is considered to be the earliest developmental stage of liver organogenesis, which coincides with the expression of albumin and AFP [57].
Once the hepatic endoderm is specified and the liver bud begins to grow, the cells become hepatoblasts and have the ability to differentiate into hepatic and biliary lineages as bipotent progenitors. Epithelial and mesenchymal cells located in the endoderm and/or mesoderm collaborate to orchestrate liver organogenesis [58] (Fig 1a). The importance of this was elegantly demonstrated in a recent in vitro study generating liver buds using induced pluripotent stem cells, human umbilical vascular endothelial cells, and mesenchymal stem cells [59].
Interaction of epithelial and mesenchymal cells in liver development and liver cancer development. a Liver bud formation is regulated by the activation of FGF, BMP, and Wnt signaling through the interaction of endodermal cells, endothelial cells, and mesenchymal cells. b Liver cancer development is regulated by the interaction of EpCAM+ and CD90+ CSCs. In primary HCC, EpCAM+ and CD90+ CSCs distinctively exist. EpCAM+ CSCs show epithelial cell features with a high tumorigenic capacity and activated Wnt signaling, whereas CD90+ CSCs show mesenchymal cell features with a highly metastatic capacity and activation of c-Kit signaling. In primary HCC where EpCAM+ and CD90+ CSCs co-exist, CD90+ CSCs regulate distant organ metastasis through the activation of TGF-β signaling, but have no effect on tumorigenicity at primary sites which is mediated by EpCAM+ CSCs
Embryogenesis and tumorigenesis share similar features including autonomous cell proliferation, motility, homing, dynamic morphologic changes, cellular heterogeneity, and interactions with the microenvironment. Liver cancer development may partially recapitulate fetal liver development in terms of the emergence of cells expressing certain stem cell markers and the activation of signaling pathways during liver development (Fig 1b). Indeed, signaling pathways activated in normal liver development are known to be activated and may be involved in the development and maintenance of liver CSCs. FGF and Wnt signaling has also been implicated in the development of HCC [60–63], with the latter shown to regulate the self-renewal of hepatoblasts and liver CSCs [20, 31, 64–68].
Moreover, as observed in the process of normal liver development, the collaboration of CSCs with epithelial or mesenchymal cell features may play an important role in the tumorigenicity and metastasis of HCC (Fig 1b). Our data indicate that EpCAM+ CSCs have no metastatic capacity for distant sites when subcutaneously injected into NOD/SCID mice. However, when CD90+ CSCs were co-injected with EpCAM+ CSCs, EpCAM+ cells could metastasize to the lung, whereas subcutaneous primary tumors showed no difference in size [36]. Furthermore, although imatinib mesylate treatment had little effect on the size of primary subcutaneous tumors, it significantly suppressed lung metastasis potentially through the suppression of CD90+ CSCs.
We found that the effect of CD90+ CSCs on the enhanced cell motility of EpCAM+ cells was mediated, at least in part, through the activation of TGF-β signaling by CD90+ CSCs (Fig 1b) [36]. This suggests that CD90+ cells are not only metastatic to the distant organ but also help the metastasis of CD90− cells, including EpCAM+ cells, which have no distant metastatic capacity of their own. Our data further suggest that imatinib mesylate inhibits distant organ metastasis by suppressing CD90+ metastatic CSCs, albeit with little effect on EpCAM+ tumorigenic epithelial stem-like CSCs, which indicates the importance of EpCAM+ and CD90+ CSC interaction in the process of HCC development, especially in distant organ metastasis. These data suggest the limitations of a treatment strategy targeting only certain CSC marker-positive cells to eradicate HCC, as it is highly possible that marker-positive CSCs exist in each HCC patient with different chemosensitivities against molecularly targeted therapy. Interestingly, we have recently identified that EpCAM+ HCC cell lines show abundant expression of the transcription factor SALL4 and high histone deacetylase activity, and the histone deacetylase inhibitor successfully suppressed proliferation of EpCAM+ HCC cell lines but showed little effect on CD90+ HCC cell lines [69]. Further studies of liver CSC heterogeneity are required to provide better treatment strategies for HCC patients.
Conclusions
There is accumulating evidence that liver CSCs play a key role in the development and perpetuation of HCC, and the importance of targeting CSCs has become clearer. Understanding the diversity of liver CSCs will further the development of personalized medicine targeting patient-specific liver CSCs.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108.
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–76.
El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340(10):745–50.
Thorgeirsson SS, Lee JS, Grisham JW. Functional genomics of hepatocellular carcinoma. Hepatology. 2006;43(2 Suppl 1):S145–50.
Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–8.
Baylin SB, Jones PA. A decade of exploring the cancer epigenome—biological and translational implications. Nat Rev Cancer. 2011;11(10):726–34.
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–61.
Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21(3):283–96.
Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. J Clin Investig. 2013;123(5):1911–8.
Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea—a paradigm shift. Cancer Res. 2006;66(4):1883–90 discussion 95-6.
Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–44.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–8.
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7.
O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–10.
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–5.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401.
Bednar F, Simeone DM. Pancreatic cancer stem cell biology and its therapeutic implications. J Gastroenterol. 2011;46(12):1345–52.
Xu G, Shen J, Ou Yang X, Sasahara M, Su X. Cancer stem cells: the ‘heartbeat’ of gastric cancer. J Gastroenterol. 2013;48(7):781–97.
Haraguchi N, Ishii H, Mimori K, Tanaka F, Ohkuma M, Kim HM, et al. CD13 is a therapeutic target in human liver cancer stem cells. J Clin Investig. 2010;120(9):3326–39.
Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136(3):1012–24.
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13(2):153–66.
Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9(1):50–63.
Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, et al. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6(7):1146–53.
Yang W, Wang C, Lin Y, Liu Q, Yu LX, Tang L, et al. OV6(+) tumor-initiating cells contribute to tumor progression and invasion in human hepatocellular carcinoma. J Hepatol. 2012;57(3):613–20.
Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44(1):240–51.
Oertel M, Shafritz DA. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta. 2008;1782(2):61–74.
Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204(8):1973–87.
Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132(7):2542–56.
Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, Desmet V. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. J Hepatol. 1998;29(3):455–63.
Van Den Heuvel MC, Slooff MJ, Visser L, Muller M, De Jong KP, Poppema S, et al. Expression of anti-OV6 antibody and anti-N-CAM antibody along the biliary line of normal and diseased human livers. Hepatology. 2001;33(6):1387–93.
Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, et al. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68(11):4287–95.
Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2008;47(2):636–47.
Qiu Q, Hernandez JC, Dean AM, Rao PH, Darlington GJ. CD24-positive cells from normal adult mouse liver are hepatocyte progenitor cells. Stem Cells Dev. 2011;20(12):2177–88.
Okada K, Kamiya A, Ito K, Yanagida A, Ito H, Kondou H, et al. Prospective isolation and characterization of bipotent progenitor cells in early mouse liver development. Stem Cells Dev. 2012;21(7):1124–33.
Xu X, Liu RF, Zhang X, Huang LY, Chen F, Fei QL, et al. DLK1 as a potential target against cancer stem/progenitor cells of hepatocellular carcinoma. Mol Cancer Ther. 2012;11(3):629–38.
Yamashita T, Honda M, Nakamoto Y, Baba M, Nio K, Hara Y, et al. Discrete nature of EpCAM(+) and CD90(+) cancer stem cells in human hepatocellular carcinoma. Hepatology. 2013;57(4):1484–97.
Zhao W, Wang L, Han H, Jin K, Lin N, Guo T, et al. 1B50-1, a mAb raised against recurrent tumor cells, targets liver tumor-initiating cells by binding to the calcium channel alpha2delta1 subunit. Cancer Cell. 2013;23(4):541–56.
Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11(2):162–71.
Huang HP, Chen PH, Yu CY, Chuang CY, Stone L, Hsiao WC, et al. Epithelial cell adhesion molecule (EpCAM) complex proteins promote transcription factor-mediated pluripotency reprogramming. J Biol Chem. 2011;286(38):33520–32.
Tang KH, Ma S, Lee TK, Chan YP, Kwan PS, Tong CM, et al. CD133(+) liver tumor-initiating cells promote tumor angiogenesis, growth, and self-renewal through neurotensin/interleukin-8/CXCL1 signaling. Hepatology. 2012;55(3):807–20.
Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387–400.
Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem cells (Dayton, Ohio). 2006;24(8):1852–8.
Sell S. The hepatocyte: heterogeneity and plasticity of liver cells. Int J Biochem Cell Biol. 2003;35(3):267–71.
Yamashita T, Honda M, Kaneko S. Heterogeneity of liver cancer stem cells. In: Wang XW, Thorgeirsson SS, Grisham JW, editors. Molecular genetics of liver neoplasia. 1st ed. New York: Springer; 2011. p. 301–17.
Jelnes P, Santoni-Rugiu E, Rasmussen M, Friis SL, Nielsen JH, Tygstrup N, et al. Remarkable heterogeneity displayed by oval cells in rat and mouse models of stem cell-mediated liver regeneration. Hepatology. 2007;45(6):1462–70.
Oertel M, Menthena A, Chen YQ, Shafritz DA. Comparison of hepatic properties and transplantation of Thy-1(+) and Thy-1(−) cells isolated from embryonic day 14 rat fetal liver. Hepatology. 2007;46(4):1236–45.
Colombo F, Baldan F, Mazzucchelli S, Martin-Padura I, Marighetti P, Cattaneo A, et al. Evidence of distinct tumour-propagating cell populations with different properties in primary human hepatocellular carcinoma. PLoS One. 2011;6(6):e21369.
Holczbauer A, Factor VM, Andersen JB, Marquardt JU, Kleiner DE, Raggi C, et al. Modeling pathogenesis of primary liver cancer in lineage-specific mouse cell types. Gastroenterology. 2013;145(1):221–31.
Chen Y, Wong PP, Sjeklocha L, Steer CJ, Sahin MB. Mature hepatocytes exhibit unexpected plasticity by direct dedifferentiation into liver progenitor cells in culture. Hepatology. 2012;55(2):563–74.
Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci USA. 2011;108(19):7950–5.
Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146(4):633–44.
Slack JM. Origin of stem cells in organogenesis. Science. 2008;322(5907):1498–501.
Calmont A, Wandzioch E, Tremblay KD, Minowada G, Kaestner KH, Martin GR, et al. An FGF response pathway that mediates hepatic gene induction in embryonic endoderm cells. Dev Cell. 2006;11(3):339–48.
Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15(15):1998–2009.
Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322(5907):1490–4.
Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442(7103):688–91.
Dabeva MD, Shafritz DA. Hepatic stem cells and liver repopulation. Semin Liver Dis. 2003;23(4):349–62.
Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39(6):1477–87.
Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–4.
Cheng AL, Shen YC, Zhu AX. Targeting fibroblast growth factor receptor signaling in hepatocellular carcinoma. Oncology. 2011;81(5–6):372–80.
Gauglhofer C, Sagmeister S, Schrottmaier W, Fischer C, Rodgarkia-Dara C, Mohr T, et al. Up-regulation of the fibroblast growth factor 8 subfamily in human hepatocellular carcinoma for cell survival and neoangiogenesis. Hepatology. 2011;53(3):854–64.
Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29(36):4989–5005.
Miura S, Mitsuhashi N, Shimizu H, Kimura F, Yoshidome H, Otsuka M, et al. Fibroblast growth factor 19 expression correlates with tumor progression and poorer prognosis of hepatocellular carcinoma. BMC Cancer. 2012;12:56.
Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67(22):10831–9.
Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68(5):1451–61.
Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47(1):288–95.
Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, et al. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50(2):472–80.
Ji J, Yamashita T, Wang XW. Wnt/beta-catenin signaling activates microRNA-181 expression in hepatocellular carcinoma. Cell Biosci. 2011;1(1):4.
Zeng SS, Yamashita T, Kondo M, Nio K, Hayashi T, Hara Y, et al. The transcription factor SALL4 regulates stemness of EpCAM-positive hepatocellular carcinoma. J Hepatol. 2014;60(1):127–34.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, T., Kaneko, S. Orchestration of hepatocellular carcinoma development by diverse liver cancer stem cells. J Gastroenterol 49, 1105–1110 (2014). https://doi.org/10.1007/s00535-014-0951-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-014-0951-1