Abstract
Purpose
We attempted to clarify the significance of atrophic change of gastric mucosa for reduction of plasma ghrelin concentration irrespective of Helicobacter pylori (Hp) infection.
Methods
Plasma acylated (acyl-)ghrelin concentration in 220 subjects, both with and without atrophic gastritis, was measured with an enzyme immunoassay kit. The extent of atrophic change of gastric mucosa was assessed and graded endoscopically. Hp infection was determined by assay of the anti-Hp antibody with an ELISA assay kit.
Results
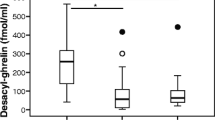
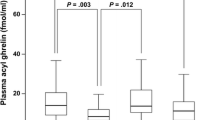
Plasma acyl-ghrelin concentration was significantly lower in the Hp positive than the Hp negative group, and Hp eradication significantly increased plasma acyl-ghrelin concentrations in Hp positive subjects. Plasma acyl-ghrelin was significantly lower in subjects with severely atrophic gastritis than in those with mild or moderate atrophic gastritis, irrespective of Hp infection or age group (<60 years old or ≧60 years old). In male subjects with a normal body mass index, plasma acyl-ghrelin concentrations in subjects with severely atrophic gastritis were significantly lower than those in subjects with mildly or moderately atrophic gastritis, suggesting that the results of the study are independent of emaciation or obesity. Logistic regression analysis showed that gastric atrophy is the key factor that modulates plasma acyl-ghrelin levels.
Conclusions
The results suggest that plasma acyl-ghrelin concentration decreases in accordance with the extent of atrophic change in gastric mucosa irrespective of Hp infection, indicating that the low plasma acyl-ghrelin level of subjects with Hp infection is mainly caused by the progress of atrophic changes in gastric mucosa.








Similar content being viewed by others
References
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a novel growth hormone releasing acylated peptide from stomach. Nature. 1999;402:656–60.
Date Y, Kojima Y, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–61.
Tscop M, Smiley DL, Heiman M. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13.
Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–8.
Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797–800.
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8.
Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–45.
Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–32.
Wren AM, Seal LA, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5.
Masuda Y, Tanaka T, Inomata M, Ohnuma N, Tanaka S, Itoh Z, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–8.
Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun. 2001;280:904–7.
Yakabi K, Shoki R, Onouchi T, Tanaka T, Ohno S, Miura S, et al. Histamine mediates the stimulatory action of ghrelin on acid secretion in rat stomach. Dig Dis Sci. 2006;56:1313–21.
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9.
Nwokolo CU, Freshwater DA, O’Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637–40.
Isomoto H, Nakazato M, Ueno H, Date y, Nishi Y, Mukae H, et al. Low plasma ghrelin levels in patients with Helicobacter pylori-associated gastritis. Am J Med. 2004;117:429–32.
Tatsuguchi A, Miyake K, Gudis K, Futagami S, Tsukui T, Wada K, et al. Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol. 2004;99:2121–7.
Osawa H, Nakazato M, Date Y, Kita H, Ohnishi H, Ueno H, et al. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metab. 2005;90:10–6.
Suzuki H, Masaoka T, Hosoda H, Nomura S, Ohara T, Kangawa K, et al. Plasma ghrelin concentration correlates with the levels of serum pepsinogen I, and pepsinogen I/II ratio—a possible novel and non-invasive marker for gastric atrophy. Hepatogastroenterology. 2004;51:1249–54.
Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, et al. Stomach is a main source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–8.
Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinhuku N, Ueta Y, et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54:8–24.
Chen C, Inui A, Asakawa A, Fujino K, Kato I, Chen C, et al. Des-acyl ghrelin acts by CRF type 2 receptors to disrupt fasted stomach motility in conscious rats. Gastroenterology. 2005;129:8–25.
Yakabi K, Kawashima J, Ono S, Ochiai M, Sakurada T, Kato S, et al. The effects of acylated ghrelin and desacyl ghrelin on acid secretion and histamine production in rat stomach. Gut suppl III. 2007;59:A235.
Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;13:87–97.
Dixon MF, Genta RM, Yardly JF, Correa P, the participants in the International Workshop on the Histopathology of Gastritis. The up-dated Sydney systems. Am J Sung Pathol. 1996;20:1161–81.
Shindler R, Ortmayer P. Classification of chronic gastritis with special reference to the gastroscopic method. Arch Intern Med. 1936;57:959–78.
Akamizu T, Murayama T, Teramukai T, Miura K, Bando I, Irako T, et al. Plasma ghrelin levels in healthy elderly volunteers: the levels of acylated ghrelin in elderly females correlate positively with serum IGF-1 levels and bowel movement frequency and negatively with systolic blood pressure. J Endocrinol. 2006;188:333–44.
Haqq AM, Farooqi IS, O’Rahilly S, Stadler DD, Rosenfeld RG, Pratt KL, et al. Serum ghrelin levels are inversely correlated with body mass index, age, and insulin concentrations in normal children and are markedly increased in Prader- Willi syndrome. J Clin Endocrinol Metab. 2003;88:174–8.
Tshop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–9.
Satoh K, Kimura K, Taniguchi Y, Yoshida Y, Kihira K, Takimoto T, et al. Distribution of inflammation and atrophy in the stomach of Helicobacter pylori-positive and negative patients with chronic gastritis. Am J Gastroenterol. 1996;91:963–9.
Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N J Med. 2002;346:1623–30.
Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, et al. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol. 2002;56:203–6.
Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–4.
Geloneze B, Tambascia MA, Pilla VF, Geloneze SR, Repetto EM, Pareja JC. Ghrelin: a gut-brain hormone: effect of gastric bypass surgery. Obesity Surg. 2003;13:17–22.
Gokcel A, Gumurdulu Y, Kayaselcuk F, Serin E, Ozer B, Ozsahin AK, et al. Helicobacter pylori has no effect on plasma ghrelin levels. Eur J Endocrinol. 2003;148:423–6.
Checci S, Montanaro M, Pasqui L, Ciuoli C, Cevenini C, Sestini F, et al. Serum ghrelin as a marker of atrophic body gastritis in patients with parietal cell antibodies. J Clin Endocrinol Metab. 2007;92:4346–51.
Rohrea GV, Welsh JD. Correlative study: gastric secretion and histology. Gastroenterology. 1967;52:185–91.
Tatsuta M, Iishi H, Okuda S. Gastric emptying and gastrointestinal symptoms in patients with atrophic gastritis and the effects of domperidone. Scand J Gastroenterol. 1989;24:251–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawashima, J., Ohno, S., Sakurada, T. et al. Circulating acylated ghrelin level decreases in accordance with the extent of atrophic gastritis. J Gastroenterol 44, 1046–1054 (2009). https://doi.org/10.1007/s00535-009-0120-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-009-0120-0