Abstract
Purpose
A Delphi study was undertaken to develop a framework guidance that would rationalise and standardise the care of children with febrile neutropenia (FNP) across the UK.
Methods
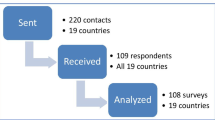
A mailed Delphi survey was undertaken with health professionals working in children's cancer units. The survey employed two rounds of feedback on 22 practice statements drawn from a systematic review of clinical evidence. Consensus was assumed for any statement where 80+ % of respondents indicated that they “agreed” or “strongly agreed”.
Results
Consensus was reached on 21 of the 22 practice statements in round 1 that were categorised into six areas: definition of fever and neutropenia, initial management and choice of antibiotic, defining low-risk patients, strategy in low-risk patients and alternative approaches. Consensus could not be reached on whether patients needed to be afebrile to be suitable for discharge and the required length of outpatient antibiotic treatment.
Conclusions
A Delphi survey allowed the successful development of a national framework for identification and management of children with FNP. The use of an existing well-functioning professional network was key in this project's success.


Similar content being viewed by others
References
Pritchard-Jones K, Kaatsch P, Steliarova-Foucher E, Stiller CA, Coebergh JWW (2006) Cancer in children and adolescents in Europe: developments over 20 years and future challenges. Eur J Cancer 42:2183–2190
Gootenberg JE, Pizzo PA (1991) Optimal management of acute toxicities of therapy. Pediatr Clin North Am 38:269–97
Hann I, Viscoli C, Paesmans M, Gaya H, Glauser M (1997) A comparison of outcome from febrile neutropenic episodes in children compared with adults: results from four EORTC studies. International Antimicrobial Therapy Cooperative Group (IATCG) of the European Organization for Research and Treatment of Cancer (EORTC). Br J Haematol 99:580–8
Chisholm JC, Dommett R (2006) The evolution towards ambulatory and day-case management of febrile neutropenia. Br J Haematol 135:3–16
Ammann RA, Hirt A, Luthy AR, Aebi C (2004) Predicting bacteremia in children with fever and chemotherapy-induced neutropenia. Pediatr Infect Dis J 23:61–7
Baorto EP, Aquino VM, Mullen CA, Buchanan GR, DeBaun MR (2001) Clinical parameters associated with low bacteremia risk in 1100 pediatric oncology patients with fever and neutropenia. Cancer 92:909–13
Klaassen RJ, Goodman TR, Pham B, Doyle JJ (2000) “Low-risk” prediction rule for pediatric oncology patients presenting with fever and neutropenia. J Clin Oncol 18:1012–9
Lucas KG, Brown AE, Armstrong D, Chapman D, Heller G (1996) The identification of febrile, neutropenic children with neoplastic disease at low risk for bacteremia and complications of sepsis. Cancer 77:791–8
Rackoff WR, Gonin R, Robinson C, Kreissman SG, Breitfeld PB (1996) Predicting the risk of bacteremia in childen with fever and neutropenia. J Clin Oncol 14:919–24
Klaassen RJMD, Allen UMD, Doyle JJMD (2000) Randomized placebo-controlled trial of oral antibiotics in pediatric oncology patients at low-risk with fever and neutropenia. SO-J Pediatr Hematol/Oncol 22(5):405–411
Paganini H, Rodriguez-Brieshcke T, Zubizarreta P, Latella A, Firpo V, Casimir L, Armada A, Fernandez C, Caceres E, Debbag R (2001) Oral ciprofloxacin in the management of children with cancer with lower risk febrile neutropenia. Cancer 91:1563–7
Petrilli AS, Dantas LS, Campos MC, Tanaka C, Ginani VC, Seber A (2000) Oral ciprofloxacin vs. intravenous ceftriaxone administered in an outpatient setting for fever and neutropenia in low-risk pediatric oncology patients: randomized prospective trial. Med Pediatr Oncol 34:87–91s
Philips R, Wade R, Stewart LA, Sutton AJ (2010) Systematic review and meta-analysis of the discriminatory performance of risk prediction rules in febrile neutropaenic episodes in children and young people. Eur J Cancer 46:2950–2964. doi:10.1016/j.ejca.2010.05.024
Philips R (2008) Predicting infectious complications in febrile neutropenic children with cancer (PICNICC) CRD 2008 available from http://www.york.ac.uk/inst/crd/projects/febrile_neutropenia_lay.htm
Phillips B, Selwood K, Lane SM, Skinner R, Gibson F, Chisholm JC (2007) Variation in policies for the management of febrile neutropenia in United Kingdom Children's Cancer Study Group centres. Arch Dis Child 92:495–8
National Institute for Health and Clinical Excellence (2005) Improving outcomes in children and young people with cancer. National Institute for Health and Clinical. Excellence, London
National Chemotherapy Advisory Group (2009) Chemotherapy services in England: ensuring quality and safety. Department of Health, London
Sung L, Phillips R, Lehrnbecher T (2011) Time for paediatric febrile neutropenia guidelines—children are not like adults. Eur J Caner 47(6):811–3
Dalkey NC, Helmer O (1963) An experimental application of the Delphi method to the use of experts. Manag Sci 9(3):458–467
Goodman CM (1987) The Delphi technique: a critique. J Adv Nurs 12(6):729–734
Keeney S, Hasson F, McKenna H (2006) Consulting the oracle: ten lessons from using the Delphi technique in nursing research. J Adv Nurs 53(2):205–212
Soanes L, Gibson F, Bayliss J, Hannan J (2000) Establishing nursing research priorities on a paediatric haemotology, oncology, infectious diseases unit: a Delphi survey. Eur J Onc Nurs 4(2):108–117
McIlfatrick S, Keeney S (2003) Cancer nursing priorities: a Delphi approach. J Adv Nurs 42(6):629–636
Morita T, Bito S, Kurihara Y, Uchitomi Y. Development of a clinical guideline for palliative sedation therapy using the Delphi method. J Palliat Med 8(4):716–729
Sowter J, Cortis J, Clarke DJ (2011) The development of evidence based guidelines for clinical practice portfolios. Nurse Ed Today (epub ahead of print)
Biondi PD, Nekolaichuk CL, Stiles C, Fainsinger R, Hagen N (2008) Applying the Delphi process to palliative care tool development: lessons learned. Support Care Cancer 16:935–942
Hasson F, Keeney S, McKenna H (2000) Research guidelines for the Delphi survey technique. J Adv Nurs 32(4):1008–1015
Centre of Evidence-based Medicine, Oxford http://www.cebm.net/index.aspx?o=1025 Accessed August 2012
Scottish Intermediate Guidelines Network (2011) SIGN 50: a guideline developers' handbook. Healthcare Improvement Scotland. http://www.sign.ac.uk/guidelines/fulltext/50/index.html Accessed January 2012
Grol R, Grimshaw J (2003) From best evidence to best practice: effective implementation of change in patients' care. Lancet 362(9391):1225–1230
Gagliardi AR, Brouwers MC, Palda VA, Lemieux-Charles L, Grimshaw J (2011) How can we improve guideline use? A conceptual framework of implementability. Implement Sci 22:6–26
Kastner M, Estey E, Bhattacharyya O (2011) Better guidelines for better care: enhancing the implementability of clinical practice guidelines. Expert Rev Pharmacoecon Outcomes Res 11(3):315–324
Grol R (2010) Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care 39(8 Suppl 2):1146–1154
Grimshaw J (2004) Implementing clinical guidelines: current evidence and future implications. J Contin Educ Health Prof 24(Suppl 1):531–537
Avery A, Savelyich BS, Sheikh A, Cantrill J, Morris CJ, Fernando B, Bainbridge M, Horsfield P, Teasdale S (2005) Identifying and establishing consensus on the most improtant safety features of FP computer systems: e-Delphi study. Inform Prim Care 13(1):3–12
Jones S, Murphy F, Edwards M, James J (2008) Doing things differently: advantages and disadvantages of web questionnaires. Nurse Res 15(4):15–26
Vehovar V, Lozar Manfreda K (2008) Internet surveys. In: de Leeuw D, Hox JJ, Dillman DA (eds) The international handbook of internet surveys. Lawrence Earlbaum, New York
Parahoo K (2008) Questionnaires. Chapter in Watson R, McKenna H, Cowman S, Keady J. Nursing research: designs and methods. Churchill Livingstone, Elsevier, Edinburgh.
Mullen CA, Petropoulos D, Roberts WM, Rytting M, Zipf T, Chan KW, Culbert SJ, Danielson M, Jeha SS, Kuttesch JF, Rolston KV (1999) Outpatient treatment of fever and neutropenia for low risk pediatric cancer patients. Cancer 86(1):126–34
Paganini H, Gomez S, Ruvinsky S, Zubizarreta P, Latella A, Fraquelli L, Iturres AS, Casimir L, Debbag R (2003) Outpatient, sequential, parenteral-oral antibiotic therapy for lower risk febrile neutropenia in children with malignant disease: A single-center, randomized, controlled trial in Argentina. Cancer 97(7):1775–80
Paul M, Soares-Weiser K, Leibovici L (2003) {beta} lactam monotherapy versus {beta} lactam-aminoglycoside combination therapy for fever with neutropenia: systematic review and meta-analysis. BMJ 326:1111
http://www.hpa.org.uk/hpa/publications/esbl_report_05/default.htm
Contopoulos-Ioannidis DG, Giotis ND, Baliatsa DV, Ioannidis JP (2004) Extended-interval aminoglycoside administration for children: A meta-analysis. Pediatrics 114(1):e111–8
Acknowledgments
The authors would like to thank the Coordinating Centre: Children's Cancer & Leukaemia Group, CCLG Data Centre, University of Leicester, Leicester, which support the CCLG Supportive Care Group. Thanks also go to CCLG centres that participated in the study.
Conflict of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix 1 Survey questions round 1
Appendix 1 Survey questions round 1
Section 1 Definition of fever and neutropenia
Febrile neutropenia (FN) is the second commonest reason for admission to paediatric oncology wards, and accounts for a considerable part of the morbidity associated with cancer in children and young adults.
Published evidence and specific UK audit data have shown reduced intensity and duration of treatment to be a safe, effective approach to therapy for certain groups of patients. However, there remains widespread variation in practice across the country. For example, most protocols in the UK use two temperature definitions:
Primary > 38.5 °C
Secondary Prolonged temperature > 38°C
Four centres vary, using primary temperatures of > 39 °C or > 38 °C (one of these centres uses a secondary temperature of > 37.5 °C twice). Most of the literature used to define risk of FN has failed to demonstrate any influence of admission temperature in defining risk of serious infection.
Many RCT and cohort studies use 38.5°C as an inclusion criterion. The EORTC (European Organisation for the Research and Treatment of Cancer) group defines “febrile” as temperature > 38.5 °C or > 38 °C twice in a twelve hour period.
There is a also range of definitions of neutropenia currently used in the UK:
ANC | n of protocols |
1 × 109/L | 14 |
0.75 × 109/L | 1 |
0.5 × 109/L or <1 × 109/L &falling | 2 |
0.5 × 109/L | 3 |
Marrow suppression has been shown to be a risk factor for infection. The EORTC definition is ' < 0.5 × 109/L or <1 × 109/L and falling'. There is considerable experience in some UK centres of safely using a cut-off of N < 0.5 × 109L as a definition of neutropenia. All current policies strongly re-enforce the concept that a significantly unwell child with malignancy should be considered functionally immunosuppressed, and antibiotic therapy commenced promptly.

Section 2 Initial management and choice of antibiotic
There are three small RCT [12, 39, 40] of out-patient therapy in paediatrics (total of 366 episodes), but they are too small to confidently define the safety of this approach.
Systematic reviews have shown no clear difference in treatment efficacy between initial carbapenem monotherapy and dual aminoglycoside/penicillin or cephalosporin combinations in paediatric febrile neutropenia. [41]. It should be noted that the UK units which use an initial montherapy (meropenem) use early addition of aminoglycoside in patients who are septic (or look unwell) as part of their management.
There is growing concern about the increasing frequency of isolation of extended spectrum beta-lactamase (ESBL) bacteria in the UK [42]. Units using primarily cephalosporin or quinolone monotherapy should pay particular attention to this issue in the local flora. A systematic review of the paediatric literature supports the use of once-daily dosing in children requiring aminoglycosides for improved bacteriological results and reduced nephrotoxicity [7].

Section 3 Defining low-risk patients
Different units may wish to compile different lists of chemotherapy protocols, which exclude patients from a low risk strategy. The majority of patients presenting in these groups will already be ineligible for low-risk treatment because of marrow suppression. It is our experience that the list of protocols is helpful for staff who are less experienced in paediatric oncology. The literature that looks at risk stratification has identified low monocyte count, signs of sepsis, and selected chemotherapy protocols as important markers of severe infection.

Section 4 Strategy in low risk patients
In order to be suitable for early discharge on oral antibiotics, patients in all units practicing risk stratification demand similar features (well, culture negative, capable family; two centres specifically exclude patients on selected 'high intensity' protocols). Out of six centres using risk stratification, four have an ANC requirement before early discharge (2 require neutrophils ≥ 0.1 × 109/L, 2 require neutrophils ≥ 0.2 × 109/l). Five centres require the patient to be afebrile (for 24 or 48 h), one specifically states that such a requirement is unnecessary.

Section 5 Outpatient antibiotic therapy
Seven centres include an option for outpatient oral antibiotic therapy in a selected low risk group after an initial period of in-hospital treatment. (In 6 of 7, this initial therapy is by intravenous antibiotics). Five protocols specify an outpatient oral antibiotic regimen to be used.
Centre | Outpatient treatment |
1 | Cefixime +/−Augmentin |
2 | Augmentin |
3 | Ciprofloxacin |
4 | Augmentin / Ciprofloxacin |
5 | Cefixime |
The protocols surveyed indicate a very large number of centres stop all antibiotic therapy in well patients after 48 hrs afebrile and cultures negative.
The published literature we have identified comparing IV and PO antibiotics has all used moderate duration total courses (5–7d). The audit data that we have from London used “until 48 h afebrile” and that from Leeds used “5 days total” therapy.
No literature has compared different durations of therapy, although it is clear that neutrophil recovery is not required for safely discontinuing antibiotics.

Section 6 Alternative approaches
Inpatient oral antibiotics in low risk patients
Oral antibiotics (Ciprofloxacin, 15 mg/kg BD) have been used from onset of therapy (in Leeds) if all the following conditions have been met (Grade B unless otherwise stated):
-
Aged >1 yr [✓]
-
No social/economic conditions that compromise access to care [✓]
-
No other medical conditions requiring hospitalisation
-
Clinically well
-
No evidence of significant source of infection (e.g. pneumonia, soft tissue infection, severe mucositis)
-
No evidence of profound marrow suppression (i.e. neutrophils ≥0.1 x109/L)
-
Not on selected intensive chemotherapy regimes (Appendix 1) [Grade D]
And they are eligible for discharge at 48 hours if additionally
-
Afebrile (for 24 hrs) [Grade D]
-
Negative blood cultures
This approach has been used for 4 years with ~50 episodes of febrile neutropenia treated in this way. No children have died or required intensive care treatment in this group.

Rights and permissions
About this article
Cite this article
Gibson, F., Chisholm, J., Blandford, E. et al. Developing a national ‘low risk’ febrile neutropenia framework for use in children and young people's cancer care. Support Care Cancer 21, 1241–1251 (2013). https://doi.org/10.1007/s00520-012-1653-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-012-1653-y