Summary
Aim
This observational study evaluated the efficacy and tolerability of 3-month aliskiren/amlodipine therapy under outpatient conditions.
Methods
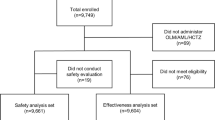
This Austria-wide observational study included 579 hypertensive patients (566 [98 %] who could be analyzed biometrically) under the care of 140 physicians. The average age of the patient collective was 64 ± 11 years and the mean duration of hypertension was 10 ± 7 years. Regarding 92 % of the study participants, an antihypertensive therapy already existed. Efficacy was assessed in accordance with the Austrian hypertension guidelines while tolerability was evaluated on the basis of adverse events. A descriptive physician judgment based on efficacy, tolerability, and compliance was available for 539 patients (95 %). Office blood pressure values were used for the evaluation.
Results
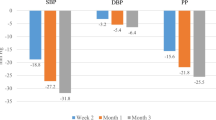
On average, the systolic and diastolic blood pressures were reduced from 161 ± 14 to 135 ± 10 mmHg and 93 ± 9 to 81 ± 6 mmHg, respectively. Blood pressure values of < 140/90 mmHg and < 130/80 mmHg were achieved in 56 and 7 % patients, respectively. A subgroup analysis of 242 patients (43 %) with diabetes mellitus and/or renal disease, as well as those with a high cardiovascular risk, demonstrated nearly identical results compared to the total population. Overall, 44 adverse events were documented in 41 patients, and physicians reported that 94 % of patients were compliant in a final survey on evaluation of therapy.
Conclusion
The fixed-dose combination of aliskiren/amlodipine provided clinically relevant blood pressure reductions along with good tolerance and compliance. During the 3-month duration of observation under outpatient conditions, it was seen that aliskiren and amlodipine reduced the systolic and diastolic blood pressures on average by 26 and 13 mmHg, respectively.




Similar content being viewed by others
References
Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ, Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360(9343):1347–60.
Statistik Austria, editors. Im Auftrag von Bundesministerium für Gesundheit, Familie und Jugend. Österreichische Gesundheitsbefragung 2006/07. Hauptergebnisse und methodische Dokumentation. Wien, 2007.
Slany J, Magometschnigg D, Mayer G, Pichler M, Pilz H, Rieder A, Schernthaner G, Skrabal F, Silberbauer K, Stoschitzky K, Watschinger B, Zweiker R. Klassifikation, Diagnostik und Therapie der Hypertonie 2007– Empfehlungen der Österreichischen Gesellschaft für Hypertensiologie. J Hyperton. 2007;11(1):7–11.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Task Force Members. ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–357.
Brown MJ, McInnes GT, Papst CC, Zhang J, MacDonald TM. Aliskiren and the calcium channel blocker amlodipine combination as an initial treatment strategy for hypertension control (ACCELERATE): a randomised, parallel-group trial. Lancet. 2011;377(9762):312–20.
Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A, VALUE trial group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–31.
Palatini P, Jung W, Shlyakhto E, Botha J, Bush C, Keefe DL. Maintenance of blood-pressure-lowering effect following a missed dose of aliskiren, irbesartan or ramipril: results of a randomized, double-blind study. J Hum Hypertens. 2010;24(2):93–103.
Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107(10):1401–6.
Drummond W, Munger MA, Rafique Essop M, Maboudian M, Khan M, Keefe DL. Antihypertensive efficacy of the oral direct renin inhibitor aliskiren as add-on therapy in patients not responding to amlodipine monotherapy. J Clin Hypertens (Greenwich). 2007;9(10):742–50.
Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J, ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906.
Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O’Rourke M, CAFÉ Investigators. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–25.
Leenen FHH, Nwachuku CE, Black HR, Cushman WC, Davis BR, Simpson LM, Alderman MH, Atlas SA, Basile JN, Cuyjet AB, Dart R, Felicetta JV, Grimm RH, Haywood LJ, Jafri SZA, Proschan MA, Thadani U, Whelton PK, Wright JT, for the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Collaborative Research Group. Clinical events in high-risk hypertensive patients randomly assigned to calcium channel blocker versus angiotensin-converting enzyme inhibitor in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Hypertension. 2006;48(3):374–84.
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23.
Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, Giampaoli S, Joffres MR, Poulter N, Primatesta P, Stegmayr B, Thamm M. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43(1):10–7.
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. Erratum in: Lancet. 2003;361(9362):1060.
Van Wijk BL, Klungel OH, Heerdink ER, de Boer A. Rate and determinants of 10-year persistence with antihypertensive drugs. J Hypertens. 2005;23(11):2101–7.
Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–30.
Friedl C, Hemetsberger M, Mader J, Fahrleitner-Pammer A, Pieber TR, Rosenkranz AR. Awareness of chronic kidney disease in Austria: a frequently under-recognized clinical picture. Wien Klin Wochenschr. 2013;125(13–14):362–7.
Fogari R, Zoppi A, Mugellini A, Maffioli P, Lazzari P, Monti C, Derosa G. Effect of aliskiren addition to amlodipine on ankle edema in hypertensive patients: a three-way crossover study. Expert Opin Pharmacother. 2011;12(9):1351–8.
Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S, ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008;372(9638):547–53.
Acknowledgments
The authors thank all patients who were willing to share their medical data. In particular, they would like to thank all participating physicians who documented these data and thereby enabled the observational study.
Conflict of interest
A.R. Rosenkranz received speakers fee from Novartis, M. Ratzinger was an employee of Novartis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosenkranz, A., Ratzinger, M. Efficacy, safety, and tolerability of antihypertensive therapy with aliskiren/amlodipine in clinical practice in Austria. The RALLY (Rasilamlo long lasting efficacy) study. Wien Klin Wochenschr 127, 203–209 (2015). https://doi.org/10.1007/s00508-015-0748-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-015-0748-0