Summary
Purpose
Intravitreal ranibizumab or bevacizumab are the most used drugs for treatment of neovascular age-related macular degeneration (nAMD). Repeated intravitreal injections represent an economic burden and may be associated with serious complications. The aim of this study is to evaluate the number of needed injections within 1 year of treatment.
Methods
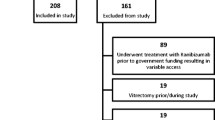
55 patients over 50 years of age with nAMD and visual acuity (VA) between 20/40 and 20/320 were included. Scheduled visits and treatment were performed monthly for 1 year. After a loading dose of three intravitreal injections (either ranibizumab = group 1 or bevacizumab = group 2), an “as needed” regimen was performed. Primary endpoint was a difference in the injection frequencies of ranibizumab and bevacizumab. Secondary endpoints were best corrected visual acuity (BCVA) and central retinal thickness (CRT).
Results
Difference in number of injections was not significant (5.00 ± 1.67 (ranibizumab group) vs. 5.80 ± 2.28 (bevacizumab group), p = 0.084). Mean BCVA was 59.12 ± 16.64 letters after 12 months if patients received ranibizumab (p = 0.001) and 64.75 ± 17.03 letters if patients received bevacizumab (p = 0.037). There was no statistical significance between the two groups (p = 0.631). The mean CRT did not differ significantly between groups after 12 months (315.67 ± 65.86 µm for ranibizumab, 350.47 ± 102.84 µm for bevacizumab, p = 0.088).
Conclusion
There was no difference in number of treatment, BCVA and CRT after 1 year between ranibizumab and bevacizumab in patients with nAMD.
Zusammenfassung
Zielsetzung
Intravitreal verabreichtes Ranibizumab oder Bevacizumab sind die am häufigsten verwendeten Medikamente in der Behandlung der neovaskulären Form der altersbedingten Makuladegeneration (nAMD). Wiederholte Verabreichung bedeutet einerseits eine ökonomische Belastung und birgt andererseits das Risiko ernsthafter Komplikationen. Ziel dieser Studie ist die Evaluierung der Anzahl der verabreichten intravitrealen Injektionen über den Zeitraum eines Jahres.
Methoden
55 Patienten über 50 Jahre mit nAMD und einem Visus zwischen 20/40 und 20/320 wurden eingeschlossen. Über den Zeitraum von 1 Jahr wurden monatliche Kontrollen und gegebenenfalls Behandlungen durchgeführt. Nach einer „loading dose“ von 3 intravitrealen Injektionen (entweder Ranibizumab = Gruppe 1 oder Bevacizumab = Gruppe 2) wurde ein „as needed“ Regime etabliert. Primärer Endpunkt war ein Unterschied in der Anzahl der Injektionen zwischen Gruppe 1 und 2. Sekundäre Endpunkte waren der best – korriegierte Visus (BCVA) und die zentrale Retinadicke (CRT).
Ergebnisse
Der Unterschied in der Anzahl der Injektionen war statistisch nicht signifikant (5,00 ± 1,67 (Ranibizumab Gruppe) zu 5,80 ± 2,28 (Bevacizumab Gruppe), p = 0,084). Der mittlere BCVA betrug 59,12 ± 16,64 Buchstaben nach 1 Jahr in Gruppe 1 (p = 0,001) und 64,75 ± 17,03 Buchstaben in Gruppe 2 (p = 0,037). Es zeigte sich keine statistische Signifikanz zwischen den beiden Gruppen (p = 0,631). Die mittlere CRT nach einem Jahr unterschied sich nicht statistisch signifikant zwischen Gruppe 1 und 2 (315,67 ± 65.86 µm bei Ranibizumab, 350,47 ± 102,84 µm bei Bevacizumab, p = 0,088).
Schlussfolgerung
Es zeigte sich kein relevanter Unterschied in der Anzahl der Behandlungen, des BCVA oder der CRT zwischen Ranibizumab und Bevacizumab bei der Behandlung der nAMD.


Similar content being viewed by others
References
Tufail A, Patel PJ, Egan C, Hykin P, da Cruz L, Gregor Z, Dowler J, Majid MA, Bailey C, Mohamed Q, Johnston R, Bunce C, Xing W. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ. 2010; doi:10.1136/bmj.c2459.
Klein R, Klein BE, Jensen SC, Mares-Pearlman JA, Cruickshanks KJ, Palta M. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106:1056–65.
Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Opthalmol. 2004;122:564–72.
Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, Reeves BC. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–411.
Jager RD, Mieler WF. Age-related macular degeneration. N Engl J Med. 2008;358:2606–17.
Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S, Shams N. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–48.
Zampros I, Praidou A, Brazitikos P, Ekonomidis P, Androudi S. Antivascular endothelial growth factor agents for neovascular age-related macular degeneration. J Ophthalmol. 2012; doi:10.1155/2012/319728.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW Jr, Esquiabro M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–83.
Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO, Weichselberger A, Staurenghi G; SUSTAIN Study Group. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118:663–71.
Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98.
Sampat KM, Garg SJ. Complications of intravitreal injections. Curr Opin Ophthalmol. 2010;21:178–83.
Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908.
Feng XF, Constable IJ, McAllister IL, Isaacs T. Comparison of visual acuity outcomes between ranibizumab and bevacizumab treatment in neovascular age-related macular degeneration. Int J Ophthalmol. 2011; doi:10.3980/j.issn.2222-3959.2011.01.20.
Acknowledgements
Andreas Scholler and Sibylla Richter-Müksch didn’t receive any unrestricted grants during the last 5 years. Birgit Weingessel and Pia Veronika Vécsei-Marlovits received unrestricted grants from Novartis Austria during the last 5 years.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scholler, A., Richter-Mueksch, S., Weingessel, B. et al. Differences of frequency in administration of ranibizumab and bevacizumab in patients with neovascular AMD. Wien Klin Wochenschr 126, 355–359 (2014). https://doi.org/10.1007/s00508-014-0539-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-014-0539-z