Abstract
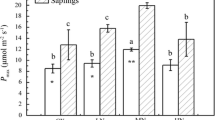
It is widely accepted that substantial nighttime sap flux (J s,n) or transpiration (E) occurs in most plants, but the physiological implications are poorly known. It has been hypothesized that J s,n or E serves to enhance nitrogen uptake or deliver oxygen; however, no clear evidence is currently available. In this study, sap flux (J s) in Eucalyptus grandis × urophylla with apparent stem photosynthesis was measured, including control trees which were covered by aluminum foil (approximately 1/3 of tree height) to block stem photosynthesis. We hypothesized that the nighttime water flux would be suppressed in trees with lower stem photosynthesis. The results showed that the green tissue degraded after 3 months, demonstrating a decrease in stem photosynthesis. The daytime J s decreased by 21.47 %, while J s,n decreased by 12.03 % in covered trees as compared to that of control, and the difference was statistically significant (P < 0.01). The linear quantile regression model showed that J s,n decreased for a given daytime transpiration water loss, indicating that J s,n was suppressed by lower stem photosynthesis in covered trees. Predawn (ψ pd) of covered trees was marginally higher than that of control while lower at predawn stomatal conductance (g s), indicating a suppressed water flux in covered trees. There was no difference in leaf carbon content and δ13C between the two groups, while leaf nitrogen content and δ15N were significantly higher in covered trees than that of the control (P < 0.05), indicating that J s,n was not used for nitrogen uptake. These results suggest that J s,n may act as an oxygen pathway since green tissue has a higher respiration or oxygen demand than non-green tissue. Thus, this study demonstrated the physiological implications of J s,n and the possible benefits of nighttime water use or E by the tree.









Similar content being viewed by others
References
Auchincloss L, Easlon HM, Levine D, Donovan L, Richards JH (2014) Pre-dawn stomatal opening does not substantially enhance early-morning photosynthesis in Helianthus annuus. Plant Cell Environ 37:1364–1370
Barbeta A, Ogaya R, Peñuelas J (2012) Comparative study of diurnal and nocturnal sap flow of Quercus ilex and Phillyrea latifolia in a Mediterranean holm oak forest in Prades (Catalonia, NE Spain). Trees 26:1651–1659
Barbour MM, Buckley TN (2007) The stomatal response to evaporative demand persists at night in Ricinus communis plants with high nocturnal conductance. Plant Cell Environ 30:711–721
Barbour MM, Cernusak LA, Whitehead D, Griffen KL, Turnbull MH, Tissue DT, Farquhar GD (2005) Nocturnal stomatal conductance and implications for modeling δ18O of leaf-respired CO2 in temperate tree species. Funct Plant Biol 32:1107–1121
Berveiller D, Kierzkowski D, Damesin C (2007) Interspecific variability of stem photosynthesis among tree species. Tree Physiol 27:53–61
Buckley TN, Turnbull TL, Pfautsch S, Adams MA (2011) Nocturnal water loss in mature subalpine Eucalyptus delegatensis tall open forests and adjacent E. pauciflora woodlands. Ecol Evol 1:435–450
Burnham KP, Anderson DR (2002) Model selection and multimodel inference, 2nd edn. Springer, New York
Cade BS, Noon BR (2003) A gentle introduction to quantile regression for ecologists. Front Ecol Environ 1:412–420
Cade BS, Noon BR, Flather CH (2005) Quantile regression reveals hidden bias and uncertainty in habitat models. Ecology 86:786–800
Caird MA, Richards JH, Hsiao TC (2007a) Significant transpirational water loss occurs throughout the night in field-grown tomato. Funct Plant Biol 34:172–177
Caird MA, Richards JH, Donovan LA (2007b) Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol 143:4–10
Cavender-Bares J, Sack L, Savage J (2007) Atmospheric and soil drought reduce nocturnal conductance in live oaks. Tree Physiol 27:611–620
Cernusak LA, Hutley LB (2011) Stable isotopes reveal the contribution of corticular photosynthesis to growth in branches of Eucalyptus miniata. Plant Physiol 155:515–523
Christman MA, Richards JH, McKay JK, Stahl EA, Juenger TE, Donovan LA (2008) Genetic variation in Arabidopsis thaliana for night-time leaf conductance. Plant Cell Environ 31:1170–1178
Christman MA, Donovan LA, Richards JH (2009a) Magnitude of nighttime transpiration does not affect plant growth or nutrition in well-watered Arabidopsis. Physiol Plant 136:264–273
Christman MA, James JJ, Drenovsky RE, Richards JH (2009b) Environmental stress and genetics influence night-time leaf conductance in the C4 grass Distichlis spicata. Funct Plant Biol 36:50–55
Craine JM, Elmore AJ, Aidar MPM, Bustamante M, Dawson TE, Hobbie EA, Kahmen A, Mack MC, McLauchlan KK, Michelsen A (2009) Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol 183:980–992
Daley MJ, Phillips NG (2006) Interspecific variation in nighttime transpiration and stomatal conductance in a mixed New England deciduous forest. Tree Physiol 26:411–419
Dawson TE, Burgess S, Tu KP, Oliveira RS, Santiago LS, Fisher JB, Simonin KA, Ambrose AR (2007) Nighttime transpiration in woody plants from contrasting ecosystems. Tree Physiol 27:561–575
de Dios VR, Turnbull MH, Barbour MM, Ontedhu J, Ghannoum O, Tissue DT (2013) Soil phosphorous and endogenous rhythms exert a larger impact than CO2 or temperature on nocturnal stomatal conductance in Eucalyptus tereticornis. Tree Physiol 33:1206–1215
Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309:630–633
Donovan LA, Richards JH, Linton MJ (2003) Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology 84:463–470
Easlon HM, Richards JH (2009) Photosynthesis affects following night leaf conductance in Vicia faba. Plant Cell Environ 32:58–63
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Ewers BE, Mackay DS, Gower ST, Ahl DE, Burrows SN, Samanta SS (2002) Tree species effects on stand transpiration in northern Wisconsin. Water Resour Res 38:1103. doi:10.1029/2001WR000830
Ewers BE, Gower ST, Bond-Lamberty B, Wang CK (2005) Effects of stand age and tree species on canopy transpiration and average stomatal conductance of boreal forests. Plant Cell Environ 28:660–678
Eyles A, Pinkard EA, O’Grady AP, Worledge D, Warren CR (2009) Role of corticular photosynthesis following defoliation in Eucalyptus globulus. Plant Cell Environ 32:1004–1014
Forster MA (2014) How significant is nocturnal sap flow? Tree Physiol 34:757–765
Fuentes S, Mahadevan M, Bonada M, Skewes MA, Cox JW (2013) Night-time sap flow is parabolically linked to midday water potential for field-grown almond trees. Irrig Sci 31:1265–1276
Gansert D (2003) Xylem sap flow as a major pathway for oxygen supply to the sapwood of birch (Betula pubescens Ehr.). Plant Cell Environ 26:1803–1814
Granier A (1987) Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol 3:309–320
Howard AR, Donovan LA (2007) Helianthus nighttime conductance and transpiration respond to soil water but not nutrient availability. Plant Physiol 143:145–155
Howard AR, Donovan LA (2010) Soil nitrogen limitation does not impact nighttime water loss in Populus. Tree Physiol 30:23–31
Hubbard RM, Stape J, Ryan MG, Almeida AC, Rojas J (2010) Effects of irrigation on water use and water use efficiency in two fast growing Eucalyptus plantations. For Ecol Manag 259:1714–1721
Koenker R (2013) Quantreg: quantile regression. R package version 5.05. [WWW document] URL http://cran.r-project.org/web/packages/quantreg/quantreg.pdf. Accessed 8 Jun 2014
Kupper P, Rohula G, Saksing L, Sellin A, Lõhmus K, Ostonena I, Helmisaari HS, Sõber A (2012) Does soil nutrient availability influence night-time water flux of aspen saplings? Environ Exp Bot 82:37–42
Lambers H, Chapin FS III, Pons JL (2008) Plant physiological ecology, 2nd edn. Springer-Verlag, Inc., New York
Lu P, Urban L, Zhao P (2004) Granier’s thermal dissipation probe (TDP) method for measuring sap flow in trees: theory and practice. Acta Bot Sin 46:631–646
Ludwig F, Jewitt RA, Donovan LA (2006) Nutrient and water addition effects on day- and night-time conductance and transpiration in a C3 desert annual. Oecologia 148:219–225
Mancuso S, Marras AM (2003) Different pathways of the oxygen supply in the sapwood of young Olea europaea trees. Planta 216:1028–1033
Marks CO, Lechowicz MJ (2007) The ecological and functional correlates of nocturnal transpiration. Tree Physiol 27:577–584
Moore GW, Cleverly JR, Owens MK (2008) Nocturnal transpiration in riparian Tamarix thickets authenticated by sap flux, eddy covariance and leaf gas exchange measurements. Tree Physiol 28:521–528
Nilsen ET, Karpa D, Mooney HA, Field C (1993) Patterns of stem photosynthesis in two invasive legumes (Spartium junceum, Cytisus scoparius) of the California coastal region. Am J Bot 80:1126–1136
Ogle K, Lucas RW, Bentley LP, Cable JM, Barron-Gafford GA, Griffith A, Ignace D, Jenerette GD, Tyler A, Huxman TE et al (2012) Differential daytime and night-time stomatal behavior in plants from North American deserts. New Phytol 194:464–476
Pfanz H, Aschan G, Langenfeld-Heyser R, Wittmann C, Loose M (2002) Ecology and ecophysiology of tree stems: corticular and wood photosynthesis. Naturwissenschaften 89:147–162
Phillips NG, Lewis JD, Logan BA, Tissue DT (2010) Inter- and intra-specific variation in nocturnal water transport in Eucalyptus. Tree Physiol 30:586–596
Poorter H, Van der Werf A, Atkin OK, Lambers H (1991) Respiratory energy requirements of roots vary with the potential growth rate of a plant species. Physiol Plant 83:469–475
Ren FF, Sun GY, Hu YB, Fan CH, Cai SY (2009) A preliminary study on photosynthetic characteristics of chlorenchyma in several tree barks. Plant Physiol J 45:249–252
Resco de Dios V, Díaz-Sierra R, Goulden ML, Barton CVM, Boer MM, Gessler A, Ferrio JP, Pfautsch S, Tissue DT (2013) Woody clockworks: circadian regulation of night-time water use in Eucalyptus globulus. New Phytol 200:743–752
Ricotta C, Godefroid S, Rocchini D (2010) Invasiveness of alien plants in Brussels is related to their phylogenetic similarity to native species. Divers Distrib 16:655–662
Rohula G, Kupper P, Räim O, Sellin A, Sõber A (2014) Patterns of night-time water use are interrelated with leaf nitrogen concentration in shoots of 16 deciduous woody species. Environ Exp Bot 99:180–188
Rosado BHP, Oliveira RS, Joly CA, Aidar MPM, Burgess SSO (2012) Diversity in nighttime transpiration behavior of woody species of the Atlantic Rain Forest, Brazil. Agric For Meteorol 158–159:13–20
Saveyn A, Steppe K, Ubierna N, Dawson TE (2010) Woody tissue photosynthesis and its contribution to trunk growth and bud development in young plants. Plant Cell Environ 33:1949–1958
Schmitz N, Egerton JJG, Lovelock CE, Ball MC (2012) Light-dependent maintenance of hydraulic function in mangrove branches: do xylary chloroplasts play a role in embolism repair? New Phytol 195:40–46
Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F (2007) Removal of nutrient limitations by long-term fertilization decreases nocturnal water loss in savanna trees. Tree Physiol 27:551–559
Snyder KA, James JJ, Richards JH, Donovan LA (2008) Does hydraulic lift or nighttime transpiration facilitate nitrogen acquisition? Plant Soil 306:159–166
Sorz J, Hietz P (2008) Is oxygen involved in beech (Fagus sylvatica) red heartwood formation? Trees 22:175–185
Tanner W, Beevers H (2001) Transpiration, a prerequisite for long-distance transport of minerals in plants? Proc Natl Acad Sci U S A 98:9443–9447
Teskey RO, Saveyn A, Steppe K, McGuire MA (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177:17–32
Vick JK, Young DR (2009) Corticular photosynthesis: a mechanism to enhance shrub expansion in coastal environments. Photosynthetica 47:26–32
Wang H, Zhao P, Cai XA, Wang Q, Ma L, Rao XQ, Zeng XP (2007) Partitioning of night sap flow of Acacia mangium and its implication for estimating whole-tree transpiration. Chin J Plant Ecol 31:777–786
Wittmann C, Pfanz H (2014) Bark and woody tissue photosynthesis a means to avoid hypoxia or anoxia in developing stem tissues. Funct Plant Biol 41:940–953
Zeppel M, Tissue D, Taylor D, Macinnis-Ng C, Eamus D (2010) Rates of nocturnal transpiration in two evergreen temperate woodland species with differing water-use strategies. Tree Physiol 30:988–1000
Zeppel MJB, Lewis JD, Chaszar B, Smith RA, Medlyn BE, Huxman TE, Tissue DT (2012) Nocturnal stomatal conductance responses to rising [CO2], temperature and drought. New Phytol 193:929–938
Zhang L, Luo TX, Deng KM, Dai Q, Huang Y, Jiang ZF, Tao MY, Zeng KY (2004) Biomass and net primary productivity of secondary evergreen broadleaved forest in Huangmian Forest Farm, Guangxi. Chin J Appl Ecol 15:2029–2033
Zhao P, Rao XQ, Ma L, Cai XA, Zeng XP (2005) Application of Granier’s sap flow system in water use of Acacia mangium forest. J Trop Subtrop Bot 13:457–468
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Grant No. 41030638, 31170673, and 41275169) and the Natural Science Foundation of Guangdong Province (Grant No. S2012020010933). We greatly acknowledge Fei Gao, Xiuhua Zhao, Zhenzhen Zhang, and Liwei Zhu for their assistance in field work. We also thank Professor Ram Oren of Duke University for his comments of the early manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, J., Zhou, J., Sun, Z. et al. Suppression of nighttime sap flux with lower stem photosynthesis in Eucalyptus trees. Int J Biometeorol 60, 545–556 (2016). https://doi.org/10.1007/s00484-015-1050-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-015-1050-6