Abstract
Background
In the absence of an ideal treatment for chronic pain associated with rheumatic diseases, there is interest in the potential effects of cannabinoid molecules, particularly in the context of global interest in the legalization of herbal cannabis for medicinal use.
Methods
A systematic search until April 2015 was conducted in Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, www.cannabis-med.org and clinicaltrials.gov for randomized controlled trials with a study duration of at least 2 weeks and at least ten patients per treatment arm with herbal cannabis or pharmaceutical cannabinoid products in fibromyalgia syndrome (FMS), osteoarthritis (OA), chronic spinal pain, and rheumatoid arthritis (RA) pain. Outcomes were reduction of pain, sleep problems, fatigue and limitations of quality of life for efficacy, dropout rates due to adverse events for tolerability, and serious adverse events for safety. The methodology quality of the randomized controlled trials (RCTs) was evaluated by the Cochrane Risk of Bias Tool.
Results
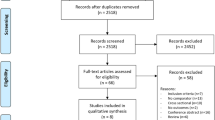
Two RCTs of 2 and 4 weeks duration respectively with nabilone, including 71 FMS patients, one 4-week trial with nabilone, including 30 spinal pain patients, and one 5-week study with tetrahydrocannbinol/cannabidiol, including 58 RA patients were included. One inclusion criterion was pain refractory to conventional treatment in three studies. No RCT with OA patients was found. The risk of bias was high for three studies. The findings of a superiority of cannabinoids over controls (placebo, amitriptyline) were not consistent. Cannabinoids were generally well tolerated despite some troublesome side effects and safe during the study duration.
Conclusions
Currently, there is insufficient evidence for recommendation for any cannabinoid preparations for symptom management in patients with chronic pain associated with rheumatic diseases.
Zusammenfassung
Hintergrund
Bei Fehlen einer optimalen Behandlung von chronischen Schmerzen bei rheumatischen Erkrankungen besteht ein Interesse in dem Potential von Cannabinoiden, insbesondere auf dem Hintergrund eines weltweiten Interesses der Legalisierung von Cannabis für medizinische Zwecke.
Methoden
Systematische Literatursuche bis April 2015 in CENTRAL, Pubmed, www.cannabis-med.org und clinicaltrials.gov nach randomisierten kontrollierten Studien (RCT) mit einer Studiendauer von mindestens zwei Wochen und mindestens 10 Patienten pro Behandlungsarm mit pflanzlichem Cannabis oder pharmazeutisch hergestellten Cannabisprodukten beim Fibromyalgiasyndrom (FMS), bei Arthrose (OA), beim Rückenschmerz und bei rheumatoider Arthritis (RA). Zielvariablen waren Reduktion von Schmerz, Müdigkeit, Schlafstörungen und Einschränkungen der Lebensqualität als Indikatoren der Wirksamkeit, Abbruchraten wegen Nebenwirkungen für Verträglichkeit und schwerwiegende Nebenwirkungen für Sicherheit. Die methodische Qualität der RCTs wurde mit dem Cochrane Risk of Bias Tool bewertet.
Ergebnisse
Zwei RCTs mit Nabilon und einer Dauer von 2 bzw. 6 Wochen mit 71 FMS –Patienten, eine 4-wöchige Studie mit Nabilon und 30 Rückenschmerzpatienten und eine 5-wöchige mit Tetrahydrocannbinol/Cannabidiol mit 58 RA-Patienten wurden eingeschlossen. Ein Einschlusskriterium in drei Studien waren Schmerzen, die auf eine konventionelle Therapie nicht ansprachen. Keine RCT mit OA-Patienten wurde gefunden. Das Verzerrungsrisiko war hoch in drei Studien. Die Ergebnisse einer Überlegenheit von Cannabinoiden gegenüber Kontrollsubstanzen (Placebo, Amitriptylin) waren nicht konsistent. Cannabinoide wurden trotz einiger unangenehmer Nebenwirkungen gut toleriert und waren sicher während der Studiendauer.
Schlussfolgerungen
Aktuell besteht keine ausreichende Evidenz, eine symptomatische Behandlung von Patienten mit chronischen Schmerzen bei rheumatischen Erkrankungen mit Cannabispräparaten zu empfehlen.


Similar content being viewed by others
References
Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE (2015) Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 162(1):46–54
Blake DR, Robson P, Ho M, Jubb RW, McCabe CS (2006) Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 45(1):50–52
Canada, D.o.J., Acts, Regulations,Health. Marihuana Medical Access Regulations (SOR/2001-227),P.C. 2001–1146 2001-06-14. http://lois-laws.justice.gc.ca/eng/regulations/SOR-2001-227/FullText.html. Accessed 01 June 2015
Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates, Hillsdale
Di Marzo V, Bifulco M, De Petrocellis L (2004) The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 3(9):771–784
Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT (2008) Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9(2):105–121
Fayers PM, Hays RD (2014) Don’t middle your MIDs: regression to the mean shrinks estimates of minimally important differences. Qual Life Res 23(1):1–4
Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M (2015) Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 14(2):162–173
Fitzcharles MA, Jamal S (2015) Expanding medical marijuana access in Canada: considerations for the rheumatologist. J Rheumatol 42(2):143–145
Fitzcharles MA, Ste-Marie PA, Goldenberg DL, Pereira JX et al (2013) 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Res Manag 18:119–126
Fitzcharles MA, Clauw DJ, Ste-Marie PA, Shir Y (2014) The dilemma of medical marijuana use by rheumatology patients. Arthritis Care Res (Hoboken) 66:797–801
Fiz J, Durán M, Capellà D, Carbonell J, Farré M (2011) Cannabis use in patients with fibromyalgia: effect on symptoms relief and health-related quality of life. PLoS One 6(4):e18440
Furukawa TA, Cipriani A, Barbui C, Brambilla P, Watanabe N (2005) Imputing response rates from means and standard deviations in meta-analyses. Int Clin Psychopharmacol 29:49–52
Guindon J, Hohmann AG (2009) The endocannabinoid system and pain. CNS Neurol Dis Drug Targets 8:403–421
Häuser W, Urrútia G, Tort S, Uçeyler N, Walitt B (2013) Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database Syst Rev 1:CD010292
Häuser W, Walitt B, Fitzcharles MA, Sommer C (2014) Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res Ther 16:201
Häuser W, Klose P, Welsch P, Petzke F, Nothacker M, Kopp I (2015) [Methodology of the development of the updated LONTS guidelines for long-term administration of opioids in noncancer pain]. Schmerz 29(1):8–34
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 87(5):1932–1936
Higgins JPT, Green S, Higgins JPT, Altman DG, Sterne JAC (editors) (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. www.cochrane-handbook.org
Hillard CJ, Weinlander KM, Stuhr KL (2012) Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience 204:207–229
Hoch E, Bonnet U, Thomasisus R, Ganzer F, Havemann-Reinecke U, Preuss UW (2015) Risks associated with the non-medical use of cannabis. Dtsch Arztebl Int 112:271–278
Landry T, Fitzcharles MA, Ste-Marie PA, Shir Y (2014) Efficacy and safety of cannabinoid treatments in the rheumatic diseases: a systematic review of randomized controlled trials. Arthritis Rheum 66(11):S110–S111
Lee MC, Ploner M, Wiech K, Bingel U et al (2013) Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain 154:124–134
Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF (2009) Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A 106(48):20270–20275
Lynch ME, Campbell F (2011) Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol 72(5):735–744
Malfait AM, Gallily R, Sumariwalla PF et al (2000) The non-psychoactive cannabis-constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA 97:9561–9566
Moher D, Liberati A, Teztlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Ann Intern Med 51:1–7
Moore RA, Barden J, Derry S, McQuay HJ (2008) Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA (eds) Systematic reviews in pain research: methodology refined. IASP Press, Seattle, pp 15–24
Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ (2010a) Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 149(2):360–364
Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S et al (2010b) “Evidence” in chronic pain—establishing best practice in the reporting of systematic reviews. Pain 150(3):386–389
National Pain Foundation (2014) Marijuana rated most effective for treating fibromyalgia. www.thenationalpainfoundation.org/pain-news.php. Accessed 1 April 2015
Office of the Information Commissioner of Canada, Information request (ATI 2013-00282) under the Access to Information Act. 2013. Accessed 01.06.2015
Petzke F, Welsch P, Klose P, Schaefert R, Sommer C, Häuser W (2015) [Opioids in chronic low back pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration]. Schmerz 29(1):60–72
Pinsger M, Schimetta W, Volc D, Hiermann E, Riederer F, Pölz W (2006) [Benefits of an add-on treatment with the synthetic cannabinomimetic nabilone on patients with chronic pain—a randomized controlled trial]. Wien Klin Wochenschr 118(11–12):327–335
Richardson D, Pearson RG, Kurian N, Latif ML, Garle MJ, Barrett DA, Kendall DA, Scammell BE, Reeve AJ, Chapman V (2008) Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res Ther 10(2):R43
Russo EB (2008) Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol Lett 29:192–200
Schaefert R, Welsch P, Klose P, Sommer C, Petzke F, Häuser W (2015) [Opioids in chronic osteoarthritis pain. A systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks duration]. Schmerz 29(1):47–59
Schley M, Legler A, Skopp G, Schmelz M, Konrad C, Rukwied R (2006) Delta-9-THC based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare, and pain relief. Curr Med Res Opin 27:1269–1276
Skrabek RQ, Galimova L, Ethans K, Perry D (2008) Nabilone for the treatment of pain in fibromyalgia. J Pain 9(2):164–173
Ste-Marie PA, Fitzcharles MA, Gamsa A, Ware MA, Shir Y (2012) Association of herbal cannabis use with negative psychosocial parameters in patients with fibromyalgia. Arthritis Care Res (Hoboken) 64(8):1202–1208
The Nordic Cochrane Centre, The Cochrane Collaboration (2014) Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen
Üçeyler N, Häuser W, Sommer C (2011) Systematic review with meta-analysis: cytokines in fibromyalgia syndrome. BMC Musculoskelet Disord 12:245
Üçeyler N, Sommer C, Walitt B, Häuser W (2013) Anticonvulsants for fibromyalgia. Cochrane Database Syst Rev 10:CD010782
van Houdenhove B, Egle UT (2004) Fibromyalgia: a stress disorder? Piecing the biopsychosocial puzzle together. Psychother Psychosom 73(5):267–275
Volkow ND, Compton WM, Weiss SR (2014) Adverse health effects of marijuana use. N Engl J Med 371(9):879
Von Korff M (2013) Opioids for chronic musculoskeletal pain: putting patient safety first. Pain 154(12):2583–2585
Ware MA, Adams H, Guy GW (2005) The medicinal use of cannabis in the UK: results of a nationwide survey. Int J Clin Pract 3:291–295
Ware MA, Fitzcharles MA, Joseph L, Shir Y (2010) The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg 10(2):604–610
Weber J, Schley M, Casutt M, Gerber H et al (2009) Tetrahydrocannabinol (Delta 9-THC) treatment in chroniccentral neuropathic pain and fibromyalgia patients: results of a multicenter survey. Anesthesiol Res Pract pii: 827290
Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J (2015) Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 313(24):2456–2473
Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL et al (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33:160–172
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P et al (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 62:600–610
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W et al (2011) Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 38:1113–1122
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.-A. Fitzcharles has received consulting fees, speaking fees and/or honoraria from ABBVIE, Abbott, Amgen, Bristol-Myers Squibb Canada, Janssen, Johnson & Johnson, Lilly, Pfizer, Purdue and Valeant. C. Baerwald has received speeking and consulting fees from Mundipharma, Grünenthal, Pfizer, MSD Sharp & Dohme and Merck. J. Ablin has no conflcits of interest to declare. W. Häuser has received speaking fees from Grünenthal, MSD Sharp & Dohme and Pfizer.
Appendices
Appendix 1
Risk of bias assessment (Cochrane Collaboration, Häuser 2015)
1. Randomization (systematic selection bias): There is a low risk of selection bias if the investigators describe the method of random allocation of patients in the therapy and control groups by the one of the following methods: referral to a random number table, use of computer-generated random numbers, coin tossing, shuffling cards or envelopes, dice throwing, or drawing lots. There is a high risk of selection bias if the allocation is generated in terms of odd or even numbers in the date of birth, date of hospital admission, or hospital record number, as well as in the case of allocation by judgment of the physician, the patient’s wishes, results of a laboratory test, or availability of the intervention.
2. Allocation concealment (selection bias): There is a low risk of systematic selection bias if the participants and investigators could not foresee allocations because one of the following methods, or an equivalent method, was used to conceal the allocation: central allocation (e.g., telephone, Internet, or pharmacy-controlled random allocation; sequentially numbered drug containers of identical appearance or sequentially numbered sealed opaque envelopes).There is a high risk of systematic selection bias if participants and investigators could possibly foresee allocations, for example due to the use of an openly available treatment plan (e.g., a list with randomly generated numbers); assignment envelopes were used without appropriate safeguards (e.g., if envelopes were unsealed, nonopaque, or not sequentially numbered); alternating or rotating treatment group allocation; date of birth; case record number; or other explicitly unconcealed allocation procedures.
3. Blinding of participants and personnel/treatment providers (systematic performance bias): There is a low risk of performance bias if blinding of participants was ensured, and it was unlikely that the blinding could have been lacking or incomplete; or if blinding was lacking or incomplete, the review authors judge that the outcome was not influenced by the lack of blinding. There is a high risk of performance bias if blinding of participants was not ensured.
4. Blinding of outcome assessor (systematic detection bias): There is low risk of systematic detection bias if the outcome assessor assessing patient-reported outcomes is not the clinical investigator but rather a statistician not involved in the treatment of the patient. There is an unclear risk of systematic detection bias if no details on the identity of the outcome assessor are reported. There is a high risk of systematic detection bias if the outcome assessor was involved in treatment of the patients.
5. Incomplete outcome data (systematic attrition bias due to loss of participants): There is low risk of systematic bias if all randomized patients were reported or analyzed in the group to which they were allocated by randomization, and dropouts were analyzed by the baseline observation carried forward (BOCF) method (baseline measurements used for data analysis). There is an unclear risk of systematic bias if all randomized patients were reported or analyzed in the group to which they were allocated by randomization, and dropouts were analyzed by the LOCF method (last measurements used for data analysis). There is a high risk of systematic bias if no intention-to-treat (ITT) analysis was carried out (analysis technique in which patients are analyzed according to their original group assignment, regardless of whether they received the intended therapy completely, in part, or not at all) or only study completers were evaluated.
6. Selective reporting (systematic reporting bias): There is low risk of reporting bias if the study protocol is available and all of the study’s prespecified primary and secondary endpoints that are of interest to the review have been reported in the prespecified way; or if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (a convincing text of this nature is probably uncommon). There is a high risk of systematic reporting bias if not all of the study’s prespecified primary outcomes have been reported; one or more primary outcomes are reported using measurements or analysis methods that were not prespecified; one or more of the reported primary outcomes were not prespecified (independently of whether justification for use of an unexpected result is provided); one or more outcomes that are of interest to the review are reported incompletely, such that they cannot be entered into meta-analysis; the study report fails to include results for a key outcome, which would be expected in a study of this nature.
7. Group similarity at baseline (systematic selection bias): There is low risk of bias if groups are similar at baseline for demographic factors, values of main outcome measures, and important prognostic factors. There is high risk of bias if groups are not similar at baseline for demographic factors, values of main outcome measures, and important prognostic factors.
Appendix 2
Characteristics of included studies (Blake 2006)
Methods
Study setting: Multi center study, no further details provided, UK
Study design: Parallel
Duration therapy: 5 weeks
Follow-up: 7–10 days
Participants: 58 (79 % women, race not reported, mean age 49 years)
Inclusion criteria
-
Diagnosis of RA meeting ACR criteria
-
Active arthritis not adequately controlled by standard medication
-
NSAID and prednisolone regimes had to have been stabilized for 1 month and DMARDs for 3 months prior to enrolment
Exclusion criteria
-
A history of psychiatric disorders or substance misuse
-
Severe cardiovascular, renal, or hepatic disorder
-
A history of epilepsy
Interventions
Active drug: Oromucosal spray, each activation delivering 2.7 mg THC and 2.5 mg CBD; starting dose was one actuation within 0.5 h of retiring, and this was increased by one actuation every 2 days to a maximum of six actuations according to individual response. Stable dosing was then maintained for a further 3 weeks; 31 participants
Placebo: 31 participants
Rescue or allowed medication: No details reported. NSAID, prednisolone, and DMARDs regimen maintained during the study
Outcomes
Pain: Daily morning pain intensity at rest and on movement (NRS 0–10); Short Form McGill Questionnaire total pain intensity
Fatigue: not assessed
Sleep: not assessed
Depression: not assessed
Anxiety: not assessed
Disability: not assessed
Health-related quality of life: not assessed
Patient-perceived improvement: not assessed
Activity scores: DAS 28
AEs: No details reported
Notes
Safety: Three serious adverse events occurred during the study in the placebo group.
Funding sources and any declaration of interest of primary investigators: supported by GW Pharmaceuticals.
Pinsger 2006
Methods
Study setting: single-center study, private clinic, Austria
Study design: crossover
Duration therapy: 4 weeks for each period, washout period 5 weeks
Follow-up: 16 weeks with free choice of study drugs
Participants: 30 (71 % women, race not reported, mean age 55 years)
Inclusion criteria
-
Chronic therapy-resistant pain in causal relationship with a pathological status of the skeletal and locomotor system
-
Pain intensity VAS > 5 despite conventional treatment with NSAIDs and/or opioids
Exclusion criteria
-
Cancer
-
Change of analgesic medication in the past 4 weeks
Interventions
Active drug: nabilone oral flexible between 0.25 and 1 mg/d, 30 participants
Placebo: 30 participants
Rescue or allowed medication: no details on rescue medication reported. NSAID and opioid regimen maintained during the study
Outcomes
Pain: change of current spine pain intensity and spine pain intensity during the past 4 weeks (Visual Analogue Scale 0–10)
Fatigue: not assessed
Sleep: not assessed
Depression: not assessed
Anxiety: not assessed
Disability: not assessed
Health-related quality of life: score of Mezzich and Cohen
Patient-perceived improvement: not assessed
AEs: no details reported
Notes
Safety: One serious adverse event occured during the study in the nabilone group.
Funding sources and any declaration of interest of primary investigators: No details reported
Skrabek 2008
Methods
Study setting: single-center study, Outpatient Musculoskeletal Rehabilitation Clinic, Canada
Study design: parallel
Duration of therapy: 4 weeks
Follow-up: 4 weeks
Participants: 40 (93 % women, race not reported, mean age 49 years)
Inclusion criteria
-
The patient meets The American College of Rheumatology (1990) criteria for the classification of fibromyalgia. [5]
-
18–70 years old.
-
Any gender.
-
The patient has not received benefit from a tricyclic antidepressant (TCA), muscle relaxant, acetaminophen, or nonsteroidal anti-inflammatories for management of their pain.
-
No previous use of oral cannabinoids for pain management.
Exclusion criteria
-
The patient’s pain is better explained by a diagnosis other than fibromyalgia.
-
Abnormalities on routine baseline blood work, including electrolytes, urea and creatinine, a complete blood count, and liver function tests (AST ALT GGT, Alk Phos, and LDH). Normal tests taken 3 months prior to the study will be accepted if there is no history of acute illness since the time the blood was drawn.
-
Heart disease (cannabinoids can reduce heart rate and blood pressure). Patients with heart disease will be excluded based on a history of angina, MI, or CHF as well as a clinical exam.
-
Schizophrenia or other psychotic disorder.
-
Severe liver dysfunction (patients will be excluded if there is an elevation of any of the baseline liver enzymes).
-
History of untreated nonpsychotic emotional disorders.
-
Cognitive impairment.
-
Major illness in another body area.
-
Pregnancy.
-
Nursing mothers.
-
Patients less than 18 years old.
-
History of drug dependency.
-
A known sensitivity to marijuana or other cannabinoid agents.
Interventions
Active drug: nabilone 0.5 mg to 1 mg/twice a day: 20 participants
Placebo: 20 participants
Rescue or allowed medication: No details reported. Subjects were asked to continue any current medication including breakthrough medications but not to begin any new therapy
Outcomes
Pain: daily diary mean pain (VAS 0–10)
Fatigue: FIQ subscale VAS 0–100
Sleep: not assessed
Depression: FIQ subscale VAS 0–100
Anxiety: FIQ subscale VAS 0–100
Disability: FIQ subscale VAS 0–100
Health-related quality of life: FIQ total score (0–100)
Patient-perceived improvement: not assessed
AEs: AEs were recorded at each visit. No details reported
Notes
Safety: no serious adverse events occurred during the study
Funding sources and any declaration of interest of primary investigators: supported by Valeant Canada and an HSC Medical Stuff Council Fellowship Fund
No declaration of interest of primary investigators included
Ware 2010
Methods
Study setting: single-center study, pain clinic, Canada
Study design: crossover
Duration therapy: 2 weeks each with 2-week washout between the two periods
Follow-up: none
Participants: 32 (81 % women, race not reported, mean age 50 years)
Inclusion criteria
Patients aged ≥ 18 years
-
A diagnosis of fibromyalgia according to the American College of Rheumatology classification criteria [51]
-
Suffering from self-reported disturbed sleep
-
Negative urine screen for cannabinoids
-
Women of childbearing potential must agree to use adequate contraception during study and for 3 months after study
-
Ability to attend research center every second week for approximately 7–9 weeks and be able to be contacted by telephone during the study period
-
Stable drug regimen for 1 month prior to randomization
-
Normal liver (AST < 3x normal) and renal function (serum creatinine < 133 µmol/L)
-
Hematocrit > 38 %
-
Negative serum bHCG
-
Proficient in English or French
-
Willing and able to give written informed consent
-
Ability to follow study protocol (cognitive and situational)
Exclusion criteria:
-
Patients currently using cannabis or cannabinoid or TCA and who are unable to undergo a 2-week washout period before entering the study
-
Pain due to cancer
-
Unstable cardiac disease such as cardiac arrhythmias, cardiac failure, ischemic heart disease, and/or hypertension on clinical history and examination
-
History of psychotic disorder or schizophrenia
-
Known hypersensitivity to cannabinoids, amitriptyline, or related TCAs
-
Currently taking or unable to stop taking monoamine oxidase inhibitors (a two-week washout period is necessary for subjects taking MAOIs)
-
History of seizures/epilepsy
-
Diagnosis of glaucoma
-
Urinary retention
-
Pregnancy and/or breast-feeding
-
Participation in other clinical trial in the 30 days prior to randomization
-
A recent manic episode (within the past year)
-
Current suicidal ideation or history of suicide attempts
Interventions
Active drug: nabilone 0.5 or 1 mg/d orally, flexible: 29 participants
Active comparator: amitriptyline oral, flexible, 10 or 20 mg/d: 29 participants
Rescue or allowed medication: no details reported
Outcomes
Pain: Mc Gill Pain Questionnaire total score
Fatigue: FIQ subscale VAS 0–100 (no details reported)
Sleep: Insomnia Severity Index (0–25) and Leeds Sleep Evaluation Questionnaire
Depression: FIQ subscale VAS 0–100
Anxiety: FIQ subscale VAS 0–100
Disability: FIQ subscale VAS 0–100
Health-related quality of life: FIQ total score (0–100)
Patient-perceived improvement: not assessed
AEs: AE were recorded at each visit by open questions to the patient (personal communication)
Notes
Safety: No serious adverse events occured during the study.
Funding sources and any declaration of interest of primary investigators: McGill Supported by the a grant of Valeant (Canada) and MC Gill University Health Center. Declaration of interest of primary investigators included
Rights and permissions
About this article
Cite this article
Fitzcharles, MA., Baerwald, C., Ablin, J. et al. Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis). Schmerz 30, 47–61 (2016). https://doi.org/10.1007/s00482-015-0084-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00482-015-0084-3
Keywords
- Cannabinoids
- Fibromyalgia syndrome
- Osteoarthritis
- Chronic spinal pain
- Rheumatoid arthritis
- Systematic review