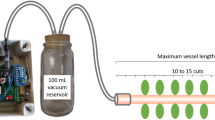
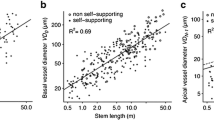
Abstract
Key message
The Cohen method of measuring vessel-length distributions is much more accurate than the DD algorithm on integer values, which should be abandoned. More research is needed to get the real distribution of vessel length.
Abstract
Scientists have been measuring the vessel length of plants for more than 50 years. The method involves infusing stem or segments with a visible substance that completely fills vessels cut open at the infusion surface. The number of infused vessels is then quantified versus distance from the infusion surface. A theoretical model is then used to convert the counts of infused vessels to a vessel length distribution. Over the years the methods and theory have changed greatly. The purpose of this review is to give the reader an understanding of why vessel length is important and to provide a theoretical basis for selection of the best method and theory to arrive at vessel length data.









Similar content being viewed by others
References
Andre JP (1998) A study of the vascular organization of bamboos (Poaceae-Bambuseae) using a microcasting method. IAWA J 19:265–278
Andre JP (2002) Organisation Vasculaire Des Angiosermes: une Vision Nouvelle. INRA, Paris
Andre JP (2005) Vascular organization of angiosperms: a new vision. Science, Enfield, NH, p 154
Bailey IW, Tupper WW (1918) Size variation in tracheary cells: 1. A comparison between the secondary xylems of vascular cryptogams, gymnosperms, and angiosperms. Am Acad Arts Sci Proc 54:149–204
Brodersen CR, Lee EF, Choat B et al (2011) Automated analysis of three-dimensional xylem networks using high-resolution computed tomography. New Phytol 191:1168–1179
Cai J, Tyree MT (2010) The impact of vessel size on vulnerability curves: data and models for within-species variability in saplings of aspen, Populus tremuloides Michx. Plant Cell Environ 33:1059–1069
Cai J, Zhang S, Tyree MT (2010) A computational algorithm addressing how vessel length might depend on vessel diameter. Plant Cell Environ 33:1234–1238
Cai J, Li S, Zhang HX, Zhang SX, Tyree MT (2014) Recalcitrant vulnerability curves: methods of analysis and the concept of fibre bridges for enhanced cavitation resistance. Plant Cell Environ 37:35–44
Cochard H, Cruiziat P, Tyree MT (1992) Use of positive pressures to establish vulnerability curves further support for the air-seeding hypothesis and implications for pressure-volume analysis. Plant Physiol 100:205–209
Cohen S, Bennink J, Tyree M (2003) Air method measurements of apple vessel length distributions with improved apparatus and theory. J Exp Bot 54:1889–1897
Comstock J, Sperry J (2000) Theoretical considerations of optimal conduit length for water transport in vascular plants. New Phytol 148:195–218
Ewers FW, Fisher JB (1989) Techniques for measuring vessel lengths and diameters in stems of woody plants. Am J Bot 76:645–656
Ewers FW, Fisher JB, Chiu S-T (1990) A survey of vessel dimensions in stems of tropical lianas and other growth forms. Oecologia 84:544–552
Greenidge KNH (1952) An approach to the study of vessel length in hardwood species. Am J Bot 39:570–574
Hacke UG, Sperry JS, Wheeler JK, Castro L (2006) Scaling of angiosperm xylem structure with safety and efficiency. Tree Physiol 26:689–701
Hacke UG, Sperry JS, Field TS (2007) Water transport in vesselless angiosperms: conducting efficiency and cavitation safety. Int J Plant Sci 168:1113–1126
Hubbard RM, Ryan MG, Stiller V, Sperry JS (2001) Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant Cell Environ 24:113–121
Jacobsen AL, Pratt RB, Tobin MF, Hacke UG, Ewers FW (2012) A global analysis of xylem vessel length in woody plants. Am J Bot 99:1583–1591
Lancashire JR, Ennos AR (2002) Modelling the hydrodynamic resistance of bordered pits. J Exp Bot 53:1485–1493
Scholander PF (1958) The rise of sap in lianas. In: Thimann KV (ed) The physiology of forest trees. Ronald, New York, pp 3–17
Skene DS, Balodis V (1968) A study of vessel length in Eucalyptus obliqua L’Herit. J Exp Bot 19:825–830
Sperry JS, Tyree MT (1988) Mechanism of water stress-induced xylem embolism. Plant Physiol 88:581–587
Sperry JS, Hacke UG, Wheeler JK (2005) Comparative analysis of end wall resistivity in xylem conduits. Plant Cell Environ 28:456–465
Tyree MT (1993) Theory of vessel-length determination: the problem of nonrandom vessel ends. Can J Bot 71:297–302
Tyree MT (1997) The cohesion–tension theory of sap ascent: current controversies. J Exp Bot 48:1753–1765
Tyree MT, Zimmermann MH (2002) Xylem structure and the ascent of sap, 2nd edn. Springer, Berlin
Wei C, Tyree MT, Steudle E (1999) Direct measurement of xylem pressure in leaves of intact maize plants: a test of the cohesion–tension theory taking hydraulic architecture into consideration. Plant Physiol 121:1191–1205
Wheeler JK, Sperry JS, Hacke UG, Hoang N (2005) Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: a basis for safety versus efficiency trade-off in xylem transport. Plant Cell Environ 28:800–812
Zimmermann MH (1971) Dicotyledonous wood structure made apparent by sequential sections (film E 1735) Inst wiss Film, Nonnenstieg 72, 34 Göttingen, Germany
Zimmermann MH (1978) Vessel ends and the disruption of water flow in plants. Phytopathology 68:253–255
Zimmermann MH (1983) Xylem structure and the ascent of sap. Springer, Berlin, Heidelberg, New York
Zimmermann MH, Brown CL (1971) Trees: structure and function. Springer, New York, Heidelberg, Berlin, p 336
Zimmermann MH, Jeje AA (1981) Vessel-length distribution in stems of some American woody plants. Can J Bot 59:1882–1892
Zimmermann MH, McDonough J (1978) Dysfunction in the flow of food. In: Horsfall JG, Cowling EB (eds) Plant disease: an advanced treatise, vol 3. Academic Press, New York, pp 117–140
Acknowledgments
The authors wish to acknowledge the following grants that made this research possible: Natural Science Foundation of China (Grant No. 31270646) to J.C. and the “thousand talent program” grants to M.T.T.
Conflict of interests
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. D. Guy.
Rights and permissions
About this article
Cite this article
Cai, J., Tyree, M.T. Measuring vessel length in vascular plants: can we divine the truth? History, theory, methods, and contrasting models. Trees 28, 643–655 (2014). https://doi.org/10.1007/s00468-014-0999-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-014-0999-9