Abstract
Background
The Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative is a quality improvement initiative to reduce dialysis-associated infections. The frequency of peritoneal dialysis (PD) catheter exit site infection (ESI) and variables influencing its development and end result are unclear. We sought to determine ESI rates, to elucidate the epidemiology, risk factors, and outcomes for ESI, and to assess for association between provider compliance with care bundles and ESI risk.
Methods
We reviewed demographic, dialysis and ESI data, and care bundle adherence and outcomes for SCOPE enrollees from October 2011 to September 2014. ESI involved only the exit site, only the subcutaneous catheter tunnel, or both.
Results
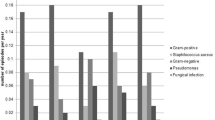
A total of 857 catheter insertions occurred in 734 children over 10,110 cumulative months of PD provided to these children. During this period 207 ESIs arose in 124 children or 0.25 ESIs per dialysis year. Median time to ESI was 392 days, with 69% of ESIs involving exit site only, 23% involving the tunnel only, and 8% involving both sites. Peritonitis developed in 6%. ESI incidence was associated with age (p = 0.003), being the lowest in children aged < 2 years and highest in those aged 6–12 years, and with no documented review of site care or an exit site score of > 0 at prior month’s visit (p < 0.001). Gender, race, end stage renal disease etiology, exit site orientation, catheter cuff number or mobilization, and presence of G-tube, stoma, or vesicostomy were unassociated with ESI incidence. Of the ESIs reported, 71% resolved with treatment, 24% required hospitalization, and 9% required catheter removal, generally secondary to tunnel infection.
Conclusions
Exit site infections occur at an annualized rate of 0.25, typically well into the dialysis course. Younger patient age and documented review of site care are associated with lower ESI rates. Although most ESIs resolve, hospitalization is frequent, and tunnel involvement/catheter loss complicate outcomes.


Similar content being viewed by others
References
Fadrowski JJAS, Warady BA (2012) The demographics of dialysis in children. In: Warady BA, Alexander SF (eds) Pediatric dialysis. Springer SBS, New York, pp 37–52
Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O'Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA (2016) US Renal Data System 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 67:Svii S1–305
North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) (2011) 2011 Annual dialysis report. Emmes Corp, Rockville
U.S. Renal Data System (USRDS) (2012) 2012 Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda
Chadha V, Schaefer FS, Warady BA (2010) Dialysis-associated peritonitis in children. Pediatr Nephrol 25:425–440
Warady BA, Sullivan EK, Alexander SR (1996) Lessons from the peritoneal dialysis patient database: a report of the North American Pediatric Renal Transplant Cooperative Study. Kidney Int Suppl 53:S68–S71
Warady BA, Bakkaloglu S, Newland J, Cantwell M, Verrina E, Neu A, Chadha V, Yap HK, Schaefer F (2012) Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int 32[Suppl 2]:S32–S86
Neu AM, Kohaut EC, Warady BA (1995) Current approach to peritoneal access in north American children: a report of the pediatric peritoneal dialysis study consortium. Adv Perit Dial 11:289–292
Schaefer F, Feneberg R, Aksu N, Donmez O, Sadikoglu B, Alexander SR, Mir S, Ha IS, Fischbach M, Simkova E, Watson AR, Moller K, von Baum H, Warady BA (2007) Worldwide variation of dialysis-associated peritonitis in children. Kidney Int 72:1374–1379
Neu AM, Miller MR, Stuart J, Lawlor J, Richardson T, Martz K, Rosenberg C, Newland J, McAfee N, Begin B, Warady BA (2014) Design of the standardizing care to improve outcomes in pediatric end stage renal disease collaborative. Pediatr Nephrol 29:1477–1484
Furth SL, Donaldson LA, Sullivan EK, Watkins SL (2000) Peritoneal dialysis catheter infections and peritonitis in children: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 15:179–182
Lloyd A, Tangri N, Shafer LA, Rigatto C, Perl J, Komenda P, Sood MM (2013) The risk of peritonitis after an exit site infection: a time-matched, case-control study. Nephrol Dial Transplant 28:1915–1921
Piraino B (1997) Infectious complications of peritoneal dialysis. Perit Dial Int 17[Suppl 3]:S15–S18
Schaefer F, Klaus G, Muller-Wiefel DE, Mehls O (1999) Intermittent versus continuous intraperitoneal glycopeptide/ceftazidime treatment in children with peritoneal dialysis-associated peritonitis. The Mid-European Pediatric Peritoneal Dialysis Study group (MEPPS). J Am Soc Nephrol 10:136–145
Hoshii S, Wada N, Honda M (2006) A survey of peritonitis and exit-site and/or tunnel infections in Japanese children on PD. Pediatr Nephrol 21:828–834
Eklund B, Honkanen E, Kyllonen L, Salmela K, Kala AR (1997) Peritoneal dialysis access: prospective randomized comparison of single-cuff and double-cuff straight Tenckhoff catheters. Nephrol Dial Transplant 12:2664–2666
Sethna CB, Bryant K, Munshi R, Warady BA, Richardson T, Lawlor J, Newland JG, Neu A (2016) Risk factors for and outcomes of catheter-associated peritonitis in children: the SCOPE collaborative. Clin J Am Soc Nephrol 11:1590–1596
van Diepen AT, Jassal SV (2013) A qualitative systematic review of the literature supporting a causal relationship between exit-site infection and subsequent peritonitis in patients with end-stage renal disease treated with peritoneal dialysis. Perit Dial Int 33:604–610
Elamin S, Khaier ME, Kaballo BG, Abu-Aisha H (2014) Low sensitivity of the exit site scoring system in detecting exit site infections in peritoneal dialysis patients. Clin Nephrol 81:100–104
Neu AM, Richardson T, Lawlor J, Stuart J, Newland J, McAfee N, Warady BA (2016) Implementation of standardized follow-up care significantly reduces peritonitis in children on chronic peritoneal dialysis. Kidney Int 89:1346–1354
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The SCOPE Consortium and its participating centers follow the Declaration of Helsinki. The SCOPE protocol was approved by an Institutional Review Board (IRB) at each center, unless a center’s IRB determined that such review was unnecessary. Informed consent was obtained on each participant in those institutions with IRB-required consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Swartz, S.J., Neu, A., Skversky Mason, A. et al. Exit site and tunnel infections in children on chronic peritoneal dialysis: findings from the Standardizing Care to Improve Outcomes in Pediatric End Stage Renal Disease (SCOPE) Collaborative. Pediatr Nephrol 33, 1029–1035 (2018). https://doi.org/10.1007/s00467-018-3889-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-3889-3