Abstract
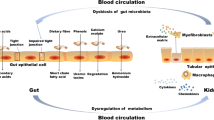
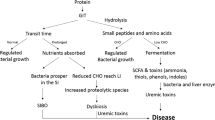
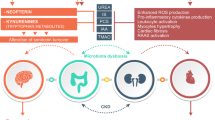
The host–gut microbiota interaction has been the focus of increasing interest in recent years. It has been determined that this complex interaction is not only essential to many aspects of normal “mammalian” physiology but that it may also contribute to a multitude of ailments, from the obvious case of inflammatory bowel disease to (complex) diseases residing in organs outside the gut. An increasing body of evidence indicates that crosstalk between host and microbiota is pathophysiologically relevant in patients with chronic kidney disease (CKD). Interactions are bidirectional; on the one hand, uremia affects both the composition and metabolism of the gut microbiota and, on the other hand, important uremic toxins originate from microbial metabolism. In addition, gut dysbiosis may induce a disruption of the epithelial barrier, ultimately resulting in increased exposure of the host to endotoxins. Due to dietary restrictions and gastrointestinal dysfunctions, microbial metabolism shifts to a predominantly proteolytic fermentation pattern in CKD. Indoxyl sulfate and p-cresyl sulfate, both end-products of protein fermentation, and trimethylamine-N-oxide, an end-product of microbial choline and carnitine metabolism, are prototypes of uremic toxins originating from microbial metabolism. The vascular and renal toxicity of these co-metabolites has been demonstrated extensively in experimental and clinical studies. These co-metabolites are an appealing target for adjuvant therapy in CKD. Treatment options include dietary therapy, prebiotics, probiotics and host and bacterial enzyme inhibitors. Final proof of the concept should come from randomized controlled and adequately powered intervention studies.

Similar content being viewed by others
References
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S (2012) host–gut microbiota metabolic interactions. Science 336:1262–1267
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012) Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108
Evenepoel P, Meijers BKI, Bammens BRM, Verbeke K (2009) Uremic toxins originating from colonic microbial metabolism. Kidney Int 76:S12–S19
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563
Graf D, Di CR, Fak F, Flint HJ, Nyman M, Saarela M, Watzl B (2015) Contribution of diet to the composition of the human gut microbiota. Microb Ecol Health Dis 26:26164
Zoetendal EG, de Vos WM (2014) Effect of diet on the intestinal microbiota and its activity. Curr Opin Gastroenterol 30:189–195
Evenepoel P, Claus D, Geypens B, Hiele M, Geboes K, Rutgeerts P, Ghoos Y (1999) Amount and fate of egg protein escaping assimilation in the small intestine of humans. Am J Physiol Gastrointest Liver Physiol 277:G935–G943
Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian MC, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR (2015) Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 6:6734
Brown JM, Hazen SL (2015) The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med 66:343–359
Cummings JH, Hill MJ, Bone ES, Branch WJ, Jenkins DJ (1979) The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr 32:2094–2101
Gabriele S, Sacco R, Altieri L, Neri C, Urbani A, Bravaccio C, Riccio MP, Iovene MR, Bombace F, De ML, Persico AM (2016) Slow intestinal transit contributes to elevate urinary p-cresol level in Italian autistic children. Autism Res 9:752–759
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI (2007) The human microbiome project. Nature 449:804–810
Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y (1996) Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 74:349–355
Wang IK, Lai HC, Yu CJ, Liang CC, Chang CT, Kuo HL, Yang YF, Lin CC, Lin HH, Liu YL, Chang YC, Wu YY, Chen CH, Li CY, Chuang FR, Huang CC, Lin CH, Lin HC (2012) Real-time PCR analysis of the intestinal microbiotas in peritoneal dialysis patients. Appl Environ Microbiol 78:1107–1112
Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, Desantis TZ, Ni Z, Nguyen TH, Andersen GL (2013) Chronic kidney disease alters intestinal microbial flora. Kidney Int 83:308–315
Yoshifuji A, Wakino S, Irie J, Tajima T, Hasegawa K, Kanda T, Tokuyama H, Hayashi K, Itoh H (2016) Gut Lactobacillus protects against the progression of renal damage by modulating the gut environment in rats. Nephrol Dial Transplant 31:401–412
Wong J, Piceno YM, Desantis TZ, Pahl M, Andersen GL, Vaziri ND (2014) Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39:230–237
Poesen R, Windey K, Neven E, Kuypers D, De Preter V, Augustijns P, D’Haese P, Evenepoel P, Verbeke K, Meijers B (2016) The influence of CKD on colonic microbial metabolism. J Am Soc Nephrol 27:1389–1399
Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P (2003) Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int 64:2196–2203
Kalantar-Zadeh K, Kopple J, Deepak S, Block D, Block G (2002) Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr 12:17–31
Wu MJ, Chang CS, Cheng CH, Chen CH, Lee WC, Hsu YH, Shu KH, Tang MJ (2004) Colonic transit time in long-term dialysis patients. Am J Kidney Dis 44:322–327
Anders HJ, Andersen K, Stecher B (2013) The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int 83:1010–1016
Vaziri ND (2012) CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens 21:587–592
Poesen R, Ramezani A, Claes K, Augustijns P, Kuypers D, Barrows IR, Muralidharan J, Evenepoel P, Meijers B, Raj DS (2015) Associations of soluble CD14 and endotoxin with mortality, cardiovascular disease, and progression of kidney disease among patients with CKD. Clin J Am Soc Nephrol 10:1525–1533
Stenvinkel P, Heimburger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T (1999) Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55:1899–1911
Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, Kieffer DA, Adams SH, Martin RJ (2014) High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One 9:e114881
Xu H, Huang X, Riserus U, Krishnamurthy VM, Cederholm T, Arnlov J, Lindholm B, Sjogren P, Carrero JJ (2014) Dietary fiber, kidney function, inflammation, and mortality risk. Clin J Am Soc Nephrol 9:2104–2110
Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, Greene T, Beddhu S (2012) High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 81:300–306
Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW (2011) Colonic contribution to uremic solutes. J Am Soc Nephrol 22:1769–1776
Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G (2009) Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA 106:3698–3703
Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368:1575–1584
Tanaka H, Sirich TL, Plummer NS, Weaver DS, Meyer TW (2015) An enlarged profile of uremic solutes. PLoS One 10:e0135657
Meijers BKI, Bammens B, Verbeke K, Evenepoel P (2008) A review of albumin binding in CKD. Am J Kidney Dis 51:839–850
Meijers BKI, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P (2010) p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol 5:1182–1189
Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA (2009) Serum indoxyl sulphate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol 4:1551–1558
Poesen R, Viaene L, Verbeke K, Claes K, Bammens B, Sprangers B, Naesens M, Vanrenterghem Y, Kuypers D, Evenepoel P, Meijers B (2013) Renal clearance and intestinal generation of p-cresyl sulphate and indoxyl sulphate in CKD. Clin J Am Soc Nephrol 8:1508–1514
Viaene L, Thijs L, Jin Y, Liu Y, Gu Y, Meijers B, Claes K, Staessen J, Evenepoel P (2014) Heritability and clinical determinants of serum indoxyl sulphate and p-cresyl sulphate, candidate biomarkers of the human microbiome enterotype. PLoS One 9:e79682
Viaene L, Annaert P, de Loor H, Poesen R, Evenepoel P, Meijers B (2013) Albumin is the main plasma binding protein for indoxyl sulphate and p-cresyl sulphate. Biopharm Drug Dispos 34:165–175
Cornelis T, Eloot S, Vanholder R, Glorieux G, van der Sande FM, Scheijen JL, Leunissen KM, Kooman JP, Schalkwijk CG (2015) Protein-bound uraemic toxins, dicarbonyl stress and advanced glycation end products in conventional and extended haemodialysis and haemodiafiltration. Nephrol Dial Transplant 30:1395–1402
Meyer TW, Peattie JW, Miller JD, Dinh DC, Recht NS, Walther JL, Hostetter TH (2007) Increasing the clearance of protein-bound solutes by addition of a sorbent to the dialysate. J Am Soc Nephrol 18:867–874
Poesen R, Evenepoel P, de Loor H, Bammens B, Claes K, Sprangers B, Naesens M, Kuypers D, Augustijns P, Meijers B (2016) The influence of renal transplantation on retained microbial-human co-metabolites. Nephrol Dial Transplant 31:1721–1729
Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G (2014) The uremic toxicity of indoxyl sulphate and p-cresyl sulphate: a systematic review. J Am Soc Nephrol 25:1897–1907
Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, Massy Z, Glorieux G, Vanholder R, Dugenet Y, Soula HA, Fouque D, Soulage CO (2013) p-Cresyl sulphate promotes insulin resistance associated with CKD. J Am Soc Nephrol 24:88–99
Asai H, Hirata J, Hirano A, Hirai K, Seki S, Watanabe-Akanuma M (2016) Activation of aryl hydrocarbon receptor mediates suppression of hypoxia-inducible factor-dependent erythropoietin expression by indoxyl sulphate. Am J Physiol Cell Physiol 310:C142–C150
Shimizu H, Bolati D, Adijiang A, Adelibieke Y, Muteliefu G, Enomoto A, Higashiyama Y, Higuchi Y, Nishijima F, Niwa T (2011) Indoxyl sulphate downregulates renal expression of Klotho through production of ROS and activation of nuclear factor-kB. Am J Nephrol 33:319–324
Adijiang A, Shimizu H, Higuchi Y, Nishijima F, Niwa T (2011) Indoxyl sulphate reduces klotho expression and promotes senescence in the kidneys of hypertensive rats. J Ren Nutr 21:105–109
Adijiang A, Niwa T (2010) An oral sorbent, AST-120, increases Klotho expression and inhibits cell senescence in the kidney of uremic rats. Am J Nephrol 31:160–164
Sun CY, Chang SC, Wu MS (2012) Suppression of Klotho expression by protein-bound uremic toxins is associated with increased DNA methyltransferase expression and DNA hypermethylation. Kidney Int 81:640–650
Muteliefu G, Shimizu H, Enomoto A, Nishijima F, Takahashi M, Niwa T (2012) Indoxyl sulphate promotes vascular smooth muscle cell senescence with upregulation of p53, p21, and prelamin A through oxidative stress. Am J Physiol Cell Physiol 303:C126–C134
Sun CY, Chang SC, Wu MS (2012) Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One 7:e34026
Meijers BK, Van KS, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, Evenepoel P (2009) The uremic retention solute p-cresyl sulphate and markers of endothelial damage. Am J Kidney Dis 54:891–901
Six I, Gross P, Remond MC, Chillon JM, Poirot S, Drueke TB, Massy ZA (2015) Deleterious vascular effects of indoxyl sulphate and reversal by oral adsorbent AST-120. Atherosclerosis 243:248–256
Niwa T, Shimizu H (2012) Indoxyl sulphate induces nephrovascular senescence. J Ren Nutr 22:102–106
Ochi A, Mori K, Nakatani S, Emoto M, Morioka T, Motoyama K, Fukumoto S, Imanishi Y, Shoji T, Ishimura E, Inaba M (2015) Indoxyl sulphate suppresses hepatic fetuin-A expression via the aryl hydrocarbon receptor in HepG2 cells. Nephrol Dial Transplant 30:1683–1692
Jing YJ, Ni JW, Ding FH, Fang YH, Wang XQ, Wang HB, Chen XN, Chen N, Zhan WW, Lu L, Zhang RY (2016) p-Cresyl sulphate is associated with carotid arteriosclerosis in hemodialysis patients and promotes atherogenesis in apoE−/− mice. Kidney Int 89:439–449
Wu CC, Hsieh MY, Hung SC, Kuo KL, Tsai TH, Lai CL, Chen JW, Lin SJ, Huang PH, Tarng DC (2016) Serum indoxyl sulphate associates with postangioplasty thrombosis of dialysis grafts. J Am Soc Nephrol 27:1254–1264
Chitalia VC, Shivanna S, Martorell J, Balcells M, Bosch I, Kolandaivelu K, Edelman ER (2013) Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation 127:365–376
Shivanna S, Kolandaivelu K, Shashar M, Belghasim M, Al-Rabadi L, Balcells M, Zhang A, Weinberg J, Francis J, Pollastri MP, Edelman ER, Sherr DH, Chitalia VC (2016) The aryl hydrocarbon receptor is a critical regulator of tissue factor stability and an antithrombotic target in uremia. J Am Soc Nephrol 27:189–201
Yang K, Wang C, Nie L, Zhao X, Gu J, Guan X, Wang S, Xiao T, Xu X, He T, Xia X, Wang J, Zhao J (2015) Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol 26:2434–2446
Sun CY, Hsu HH, Wu MS (2013) p-Cresol sulphate and indoxyl sulphate induce similar cellular inflammatory gene expressions in cultured proximal renal tubular cells. Nephrol Dial Transplant 28:70–78
Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, Perdew GH (2010) The uremic toxin 3-indoxyl sulphate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 49:393–400
Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y (2006) Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 69:1081–1087
Meijers BKI, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P (2008) Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73:1174–1180
Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA, on behalf of the European Uraemic Toxin Work Group (EUTox) (2010) Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25:1183–1191
Niewczas MA, Sirich TL, Mathew AV, Skupien J, Mohney RP, Warram JH, Smiles A, Huang X, Walker W, Byun J, Karoly ED, Kensicki EM, Berry GT, Bonventre JV, Pennathur S, Meyer TW, Krolewski AS (2014) Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study. Kidney Int 85:1214–1224
Lin CJ, Wu V, Wu PC, Wu CJ (2015) Meta-analysis of the associations of p-cresyl sulphate (P-CRESYL SULPHATE) and indoxyl sulphate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One 10:e0132589
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL (2013) Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19:576–585
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63
Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL (2015) Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 116:448–455
Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, Schmidt K, Gupta A, Wetmore JB, Nolin TD, Spertus JA, Yu AS (2016) Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 27:305–313
Missailidis C, Hallqvist J, Qureshi AR, Barany P, Heimburger O, Lindholm B, Stenvinkel P, Bergman P (2016) Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One 11:e0141738
Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL (2016) Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165:111–124
Schloerb PR (1990) Intestinal dialysis for kidney failure. Personal experience. ASAIO Trans 36:4–7
Twiss EE, Kolff WJ (1951) Treatment of uremia by perfusion of an isolated intestinal loop; survival for forty-six days after removal of the only functioning kidney. J Am Med Assoc 146:1019–1022
Poesen R, Meijers B, Evenepoel P (2013) The colon: an overlooked site for therapeutics in dialysis patients. Semin Dial 26:323–332
Evenepoel P, Meijers BK (2012) Dietary fiber and protein: nutritional therapy in chronic kidney disease and beyond. Kidney Int 81:227–229
Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Leotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A (2010) Prebiotic effects: metabolic and health benefits. Br J Nutr 104[Suppl 2]:S1–S63
Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P (2010) p-Cresyl sulphate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant 25:219–224
Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW (2014) Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol 9:1603–1610
Salmean YA, Segal MS, Palii SP, Dahl WJ (2015) Fiber supplementation lowers plasma p-cresol in chronic kidney disease patients. J Ren Nutr 25:316–320
Patel KP, Luo FJ, Plummer NS, Hostetter TH, Meyer TW (2012) The production of p-cresol sulphate and indoxyl sulphate in vegetarians versus omnivores. Clin J Am Soc Nephrol 7:982–988
Marier JF, Guilbaud R, Kambhampati SRP, Mathew P, Moberly J, Lee J, Salazar DE (2006) The effect of AST-120 on the single-dose pharmacokinetics of losartan and losartan acid (E-3174) in healthy subjects. J Clin Pharmacol 46:310–320
Schulman G, Agarwal R, Acharya M, Berl T, Blumenthal S, Kopyt N (2006) A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am J Kidney Dis 47:565–577
Niwa T, Nomura T, Sugiyama S, Miyazaki T, Tsukushi S, Tsutsui S (1997) The protein metabolite hypothesis, a model for the progression of renal failure: an oral adsorbent lowers indoxyl sulphate levels in undialyzed uremic patients. Kidney Int 52:S23–S28
Niwa T, Ise M, Miyazaki T, Meada K (1993) Suppressive effect of an oral sorbent on the accumulation of p-cresol in the serum of experimental uremic rats. Nephron 65:82–87
Yamagishi S, Nakamura K, Matsui T, Inoue H, Takeuchi M (2007) Oral administration of AST-120 (Kremezin) ia a promizing therapeutic strategy for advanced glycation end product (AGE)-related disorders. Med Hypotheses 69:666–668
Owada K, Nakao M, Koike J, Ujiie K, Tomita K, Shiigai T (1997) Effects of oral adsorbent AST-120 on the progression of chronic renal failure: a randomized controlled study. Kidney Int 63:188–190
Shoji T, Wada A, Inoue K, Hayashi D, Tomida K, Furumatsu Y, Kaneko T, Okada N, Fukuhara Y, Imai E, Tsubakihara Y (2007) Prospective randomized study evaluating the efficacy of the spherical adsorptive carbon AST-120 in chronic kidney disease patients with moderate decrease in renal function. Nephron Clin Pract 105:C99–C107
Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Arita K, Kato A, Shimizu M (2015) Randomized placebo-controlled EPPIC Trials of AST-120 in CKD. J Am Soc Nephrol 26:1732–1746
de Smet R, Thermote F, Lameire N, Vanholder R (2004) Sevelamer hydrochloride (Renagel) absorbs the uremic compounds indoxyl sulphate, indole and p-cresol [Abstract]. J Am Soc Nephrol 15:505A (abstract)
Phan O, Ivanovski O, Nguyen-Khoa T, Mothu N, Angulo J, Westenfeld R, Ketteler M, Meert N, Maizel J, Nikolov IG, Vanholder R, Lacour B, Drueke TB, Massy ZA (2005) Sevelamer prevents uremia-enhanced atherosclerosis progression in apolipoprotein E-deficient mice. Circulation 112:2875–2882
Brandenburg VM, Schlieper G, Heussen N, Holzmann S, Busch B, Evenepoel P, Vanholder R, Meijers B, Meert N, Fassbender WJ, Floege J, Jahnen-Dechent W, Ketteler M (2010) Serological cardiovascular and mortality risk predictors in dialysis patients receiving sevelamer: a prospective study. Nephrol Dial Transplant 25:2672–2679
Koppe L, Mafra D, Fouque D (2015) Probiotics and chronic kidney disease. Kidney Int 88:958–966
Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL (2015) Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163:1585–1595
Poesen R, Meijers B, Evenepoel P (2016) Adverse effects of proton pump inhibitors in chronic kidney disease. JAMA Intern Med 176:867–868
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Evenepoel, P., Poesen, R. & Meijers, B. The gut–kidney axis. Pediatr Nephrol 32, 2005–2014 (2017). https://doi.org/10.1007/s00467-016-3527-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-016-3527-x