Abstract
Background
Arterial blood supply deficiency and venous congestion both play a role in anastomotic complications. Our aim was to evaluate a software-based analysis of the fluorescence signal to recognize the patterns of bowel ischemia.
Methods
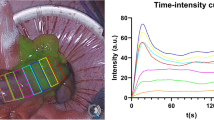
In 18 pigs, two clips were applied on the inferior mesenteric artery (group A: n = 6) or vein (group V: n = 6) or on both (group A–V: n = 6). Three regions of interest (ROIs) were identified on the sigmoid: P = proximal to the first clip; C = central, between the two clips; and D = distal to the second clip. Indocyanine Green was injected intravenously. The fluorescence signal was captured by means of a near-infrared laparoscope. The time-to-peak (seconds) and the maximum fluorescence intensity were recorded using software. A normalized fluorescence intensity unit (NFIU: 0-to-1) was attributed, using a reference card. The NFIU’s over-time variations were computed every 10 min for 50 min. Capillary lactates were measured on the sigmoid at the 3 ROIs. Various machine learning algorithms were applied for ischemia patterns recognition.
Results
The time-to-peak at the ischemic ROI C was significantly longer in group A versus V (20.1 ± 13 vs. 8.43 ± 3.7; p = 0.04) and in group A–V versus V (20.71 ± 11.6 vs. 8.43 ± 3.7; p = 0.03). The maximal NIFU at ROI C, was higher in the V group (1.01 ± 0.21) when compared to A (0.61 ± 0.11; p = 0.002) and A–V (0.41 ± 0.2; p = 0.0005). Capillary lactates at ROI C were lower in V (1.3 ± 0.6) than in A (1.9 ± 0.5; p = 0.0071), and A–V (2.6 ± 1.5; p = 0.034). The K nearest neighbor and the Linear SVM algorithms provided both an accuracy of 75% in discriminating between A versus V and 85% in discriminating A versus A–V. The accuracy dropped to 70% when the ML had to identify the ROI and the type of ischemia simultaneously.
Conclusions
The computer-assisted dynamic analysis of the fluorescence signal enables the discrimination between different bowel ischemia models.






Similar content being viewed by others
Change history
17 April 2019
In the original version, Ines Gockel was omitted as a coauthor. The complete author listing is corrected here.
References
van Manen L, Handgraaf HJM, Diana M, Dijkstra J, Ishizawa T, Vahrmeijer AL, Mieog JSD (2018) A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J Surg Oncol 118:283–300
Degett TH, Andersen HS, Gogenur I (2016) Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbecks Arch Surg 401:767–775
Blanco-Colino R, Espin-Basany E (2018) Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol 22:15–23
Ohi M, Toiyama Y, Mohri Y, Saigusa S, Ichikawa T, Shimura T, Yasuda H, Okita Y, Yoshiyama S, Kobayashi M, Araki T, Inoue Y, Kusunoki M (2017) Prevalence of anastomotic leak and the impact of indocyanine green fluorescein imaging for evaluating blood flow in the gastric conduit following esophageal cancer surgery. Esophagus 14:351–359
Karampinis I, Keese M, Jakob J, Stasiunaitis V, Gerken A, Attenberger U, Post S, Kienle P, Nowak K (2018) Indocyanine green tissue angiography can reduce extended bowel resections in acute mesenteric ischemia. J Gastrointest Surg. https://doi.org/10.1007/s11605-018-3855-1
Liot E, Assalino M, Buchs NC, Schiltz B, Douissard J, Morel P, Ris F (2018) Does near-infrared (NIR) fluorescence angiography modify operative strategy during emergency procedures? Surg Endosc. https://doi.org/10.1007/s00464-018-6226-9
Diana M, Agnus V, Halvax P, Liu YY, Dallemagne B, Schlagowski AI, Geny B, Diemunsch P, Lindner V, Marescaux J (2015) Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg 102:e169–e176
Diana M, Dallemagne B, Chung H, Nagao Y, Halvax P, Agnus V, Soler L, Lindner V, Demartines N, Diemunsch P, Geny B, Swanstrom L, Marescaux J (2014) Probe-based confocal laser endomicroscopy and fluorescence-based enhanced reality for real-time assessment of intestinal microcirculation in a porcine model of sigmoid ischemia. Surg Endosc 28:3224–3233
Diana M, Halvax P, Dallemagne B, Nagao Y, Diemunsch P, Charles AL, Agnus V, Soler L, Demartines N, Lindner V, Geny B, Marescaux J (2014) Real-time navigation by fluorescence-based enhanced reality for precise estimation of future anastomotic site in digestive surgery. Surg Endosc 28:3108–3118
Diana M, Noll E, Agnus V, Liu YY, Kong SH, Legner A, Diemunsch P, Marescaux J (2017) Reply to letter: “enhanced reality fluorescence videography to assess bowel perfusion: the cybernetic eye”. Ann Surg 265:e49–e52
Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V, Soler L, Barry B, Namer IJ, Demartines N, Charles AL, Geny B, Marescaux J (2014) Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 259:700–707
Lee KT, Mun GH (2017) Benefits of superdrainage using SIEV in DIEP flap breast reconstruction: a systematic review and meta-analysis. Microsurgery 37:75–83
Fujioka M, Hayashida K, Fukui K, Ishiyama S, Saijo H, Taniguchi K (2017) Venous superdrained gastric tube pull-up procedure for hypopharyngeal and cervical esophageal reconstruction reduces postoperative anastomotic leakage and stricture. Dis Esophagus 30:1–6
Wang WL (2017) Venous congestion in ischemic bowel. N Engl J Med 377:e10
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. J Gene Med 12:561–563
Diana M, Halvax P, Pop R, Schlagowski I, Bour G, Liu YY, Legner A, Diemunsch P, Geny B, Dallemagne B, Beaujeux R, Demartines N, Marescaux J (2015) Gastric supply manipulation to modulate ghrelin production and enhance vascularization to the cardia: proof of the concept in a porcine model. Surg Innov 22:5–14
Diana M, Noll E, Legner A, Kong SH, Liu YY, Schiraldi L, Marchegiani F, Bano J, Geny B, Charles AL, Dallemagne B, Lindner V, Mutter D, Diemunsch P, Marescaux J (2018) Impact of valve-less vs. standard insufflation on pneumoperitoneum volume, inflammation, and peritoneal physiology in a laparoscopic sigmoid resection experimental model. Surg Endosc 32:3215–3224
Pedregosa F, Gaël V, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, Vanderplas J, Passos A, Cournapeau D, Brucher M, Perrot M, Duchesnay É (2011) Scikit-learn: machine learning in python. J Mach Learn Res 12:2825–2830
Devijver PA, Kittler J (1982) Pattern recognition: a statistical approach. Prentice-Hall, London
Ris F, Liot E, Buchs NC, Kraus R, Ismael G, Belfontali V, Douissard J, Cunningham C, Lindsey I, Guy R, Jones O, George B, Morel P, Mortensen NJ, Hompes R, Cahill RA, Near-Infrared Anastomotic Perfusion Assessment Network V (2018) Multicentre phase II trial of near-infrared imaging in elective colorectal surgery. Br J Surg 105:1359–1367
Armstrong G, Croft J, Corrigan N, Brown JM, Goh V, Quirke P, Hulme C, Tolan D, Kirby A, Cahill R, O’Connell PR, Miskovic D, Coleman M, Jayne D (2018) IntAct: intra-operative fluorescence angiography to prevent anastomotic leak in rectal cancer surgery: a randomized controlled trial. Colorectal Dis. https://doi.org/10.1111/codi.14257
Diana M (2017) Enabling precision digestive surgery with fluorescence imaging. Transl Gastroenterol Hepatol 2:97
Diana M (2018) Fluorescence-guided surgery applied to the digestive system: the cybernetic eye to see the invisible. Cir Esp 96:65–68
Koyanagi K, Ozawa S, Oguma J, Kazuno A, Yamazaki Y, Ninomiya Y, Ochiai H, Tachimori Y (2016) Blood flow speed of the gastric conduit assessed by indocyanine green fluorescence: new predictive evaluation of anastomotic leakage after esophagectomy. Medicine (Baltimore) 95:e4386
Wada T, Kawada K, Takahashi R, Yoshitomi M, Hida K, Hasegawa S, Sakai Y (2017) ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surgical endoscopy 31:4184–4193
Selka F, Agnus V, Nicolau S, Bessaid A, Soler L, Marescaux J, Diana M (2014) Fluorescence-based enhanced reality for colorectal endoscopic surgery. Biomedical Image Registration, London, pp 114–123
Diana M, Noll E, Diemunsch P, Moussallieh FM, Namer IJ, Charles AL, Lindner V, Agnus V, Geny B, Marescaux J (2015) Metabolism-guided bowel resection: potential role and accuracy of instant capillary lactates to identify the optimal resection site. Surg Innov 22:453–461
Diana M, Pop R, Beaujeux R, Dallemagne B, Halvax P, Schlagowski I, Liu YY, Diemunsch P, Geny B, Lindner V, Marescaux J (2015) Embolization of arterial gastric supply in obesity (EMBARGO): an endovascular approach in the management of morbid obesity. proof of the concept in the porcine model. Obes Surg 25:550–558
Cruz RJ Jr, Garrido AG, Ribeiro CM, Harada T, Rocha-e-Silva M (2010) Regional blood flow distribution and oxygen metabolism during mesenteric ischemia and congestion. J Surg Res 161:54–61
Guzman-de la Garza FJ, Camara-Lemarroy CR, Alarcon-Galvan G, Cordero-Perez P, Munoz-Espinosa LE, Fernandez-Garza NE (2009) Different patterns of intestinal response to injury after arterial, venous or arteriovenous occlusion in rats. World J Gastroenterol 15:3901–3907
Yano K, Hata Y, Matsuka K, Ito O, Matsuda H (1994) Time limits for intestinal ischemia and congestion: an experimental study in rats. Ann Plast Surg 32:310–314
Kimura M, Kataoka M, Kuwabara Y, Sato A, Sugiura M, Fujii Y (2003) Real-time energy metabolism of intestine during arterial versus venous occlusion in the rat. J Gastroenterol 38:849–853
Vincenti M, Behrends M, Dang K, Park YH, Hirose R, Blasi-Ibanez A, Liu T, Serkova NJ, Niemann CU (2010) Induction of intestinal ischemia reperfusion injury by portal vein outflow occlusion in rats. J Gastroenterol 45:1103–1110
Nasser A, Fourman MS, Gersch RP, Phillips BT, Hsi HK, Khan SU, Gelfand MA, Dagum AB, Bui DT (2015) Utilizing indocyanine green dye angiography to detect simulated flap venous congestion in a novel experimental rat model. J Reconstr Microsurg 31:590–596
Bodin F, Diana M, Koutsomanis A, Robert E, Marescaux J, Bruant-Rodier C (2015) Porcine model for free-flap breast reconstruction training. J Plast Reconstr Aesthet Surg 68:1402–1409
Milstein DM, Ince C, Gisbertz SS, Boateng KB, Geerts BF, Hollmann MW, van Berge Henegouwen MI, Veelo DP (2016) Laser speckle contrast imaging identifies ischemic areas on gastric tube reconstructions following esophagectomy. Medicine (Baltimore) 95:e3875
Diana M, Hubner M, Vuilleumier H, Bize P, Denys A, Demartines N, Schafer M (2011) Redistribution of gastric blood flow by embolization of gastric arteries before esophagectomy. Ann Thorac Surg 91:1546–1551
Yoshimi F, Asato Y, Ikeda S, Okamoto K, Komuro Y, Imura J, Itabashi M (2006) Using the supercharge technique to additionally revascularize the gastric tube after a subtotal esophagectomy for esophageal cancer. Am J Surg 191:284–287
Kono K, Sugai H, Omata H, Fujii H (2007) Transient bloodletting of the short gastric vein in the reconstructed gastric tube improves gastric microcirculation during esophagectomy. World J Surg 31:780–784 (discussion 785–786)
Acknowledgements
Authors are grateful to Christopher Burel, professional in Medical English proofreading for his assistance with the manuscript revision.
Funding
This study was funded by the ARC Foundation for Cancer Research, a French foundation entirely dedicated to cancer research, in the framework of a large project (ELIOS: Endoscopic Luminescent Imaging for precision Oncologic Surgery) aiming at the development of fluorescence-guided surgery. https://www.fondation-arc.org/projets/ameliorer-diagnostic-et-traitement-chirurgical-cancers-digestifs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Michele Diana is the recipient of the ELIOS grant from the ARC foundation. Jacques Marescaux is the President of both IRCAD and IHU-Strasbourg Institutes, which are partly funded by Karl Storz, Medtronic and Siemens Healthcare. Giuseppe Quero, Alfonso Lapergola, Manuel Barberio, Barbara Seeliger, Ines Gockel, Paola Saccomandi, Ludovica Guierriero, Didier Mutter, Alend Saadi, Marc Worreth and Vincent Agnus have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Quero, G., Lapergola, A., Barberio, M. et al. Discrimination between arterial and venous bowel ischemia by computer-assisted analysis of the fluorescent signal. Surg Endosc 33, 1988–1997 (2019). https://doi.org/10.1007/s00464-018-6512-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6512-6