Abstract
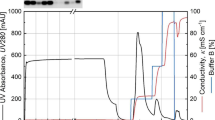
The demand for plasmid DNA (pDNA) has increased in response to the rapid advances in vaccines applications to prevent and treat infectious diseases caused by virus, bacteria or parasites, such as Leishmania species. The immunization protocols require large amounts of supercoiled plasmid DNA (sc-pDNA) challenging the development of efficient and profitable processes for capturing and purified pDNA molecules from large volumes of lysates. A typical bioprocess involves four steps: fermentation, primary recovery, intermediate recovery and final purification. Ion-exchange chromatography is one of the key operations in the purification schemes of pDNA owing the chemical structure of these macromolecules. The goal of this research was to compare the performance of the final purification step of pDNA using ion-exchange chromatography on columns packed with Mustang Q membranes or perfusive beads POROS 50 HQ. The experimental results showed that both matrixes could separate the plasmid pVAX1-NH36 (3936 bp) from impurities in clarified Escherichia coli lysates with an adequate resolution. In addition, a 24- and 21-fold global purification factor was obtained. An 88 and 63% plasmid recuperation was achieved with ion-exchange membranes and perfusion beads, respectively. A better understanding of perfusion-based matrices for the purification of pDNA was developed in this research.




Similar content being viewed by others
References
Kutzler MA, Weiner DB (2008) DNA vaccines: ready for prime time? Nat Rev Genet 9(10):776–788. doi:10.1038/nrg2432
Blaese M, Blankenstein T, Brenner M, Cohen-Haguenauer O, Gansbacher B, Russell S, Sorrentino B, Velu T (1995) Vectors in cancer therapy: how will they deliver? Cancer Gene Ther 2(4):291–297
Montgomery DL, Ulmer JB, Donnelly JJ, Liu MA (1997) DNA vaccines. Pharmacol Ther 74(2):195–205
Mountain A (2000) Gene therapy: the first decade. Trends Biotechnol 18(3):119–128
Christie RJ, Nishiyama N, Kataoka K (2010) Delivering the code: polyplex carriers for deoxyribonucleic acid and ribonucleic acid interference therapies. Endocrinology 151(2):466–473. doi:10.1210/en.2009-1045
Brave A, Ljungberg K, Wahren B, Liu MA (2007) Vaccine delivery methods using viral vectors. Mol Pharm 4(1):18–32. doi:10.1021/mp060098+
Sousa F, Prazeres DM, Queiroz JA (2008) Affinity chromatography approaches to overcome the challenges of purifying plasmid DNA. Trends Biotechnol 26(9):518–525. doi:10.1016/j.tibtech.2008.05.005
Zhong L, Scharer J, Moo-Young M, Fenner D, Crossley L, Honeyman CH, Suen SY, Chou CP (2011) Potential application of hydrogel-based strong anion-exchange membrane for plasmid DNA purification. J Chromatogr B Analyt Technol Biomed Life Sci 879(9–10):564–572. doi:10.1016/j.jchromb.2011.01.017
Nasir A (2009) Nanotechnology in vaccine development: a step forward. J Invest Dermatol 129(5):1055–1059. doi:10.1038/jid.2009.63
Kedzierski L (2011) Leishmaniasis. Human Vaccines 7(11):1204–1214. doi:10.4161/hv.7.11.17752
Palatnik-de-Sousa CB (2008) Vaccines for leishmaniasis in the fore coming 25 years. Vaccine 26(14):1709–1724. doi:10.1016/j.vaccine.2008.01.023
Nagill R, Kaur S (2011) Vaccine candidates for leishmaniasis: a review. Int Immunopharmacol 11(10):1464–1488. doi:10.1016/j.intimp.2011.05.008
Stadler J, Lemmens R, Nyhammar T (2004) Plasmid DNA purification. J Gene Med 6(Suppl 1):S54–S66. doi:10.1002/jgm.512
Ghanem A, Healey R, Adly FG (2013) Current trends in separation of plasmid DNA vaccines: a review. Anal Chim Acta 760:1–15. doi:10.1016/j.aca.2012.11.006
Schleef M, Schmidt T (2004) Animal-free production of ccc-supercoiled plasmids for research and clinical applications. J Gene Med 6(Suppl 1):S45–S53. doi:10.1002/jgm.511
Pereira LR, Prazeres DM, Mateus M (2010) Hydrophobic interaction membrane chromatography for plasmid DNA purification: design and optimization. J Sep Sci 33(9):1175–1184. doi:10.1002/jssc.200900844
Diogo MM, Queiroz JA, Prazeres DM (2005) Chromatography of plasmid DNA. J Chromatogr A 1069(1):3–22
Carbone A, Fioretti FM, Fucci L, Ausio J, Piscopo M (2012) High efficiency method to obtain supercoiled DNA with a commercial plasmid purification kit. Acta Biochim Pol 59(2):275–278
Yang Y, Hebron HR, Hang J (2008) High performance DNA purification using a novel ion exchange matrix. J Biomol Tech 19(3):205–210
Chang C-S, Ni H-S, Suen S-Y, Tseng W-C, Chiu H-C, Chou CP (2008) Preparation of inorganic–organic anion-exchange membranes and their application in plasmid DNA and RNA separation. J Membr Sci 311(1–2):336–348. doi:10.1016/j.memsci.2007.12.034
Matos T, Queiroz JA, Bulow L (2014) Plasmid DNA purification using a multimodal chromatography resin. J Mol Recognit 27(4):184–189. doi:10.1002/jmr.2349
Prazeres DMF (2011) Plasmid biopharmaceuticals: basics, applications, and manufacturing. Wiley, Hoboken, NJ
Teeters MA, Conrardy SE, Thomas BL, Root TW, Lightfoot EN (2003) Adsorptive membrane chromatography for purification of plasmid DNA. J Chromatogr A 989(1):165–173
Ghosh R (2002) Protein separation using membrane chromatography: opportunities and challenges. J Chromatogr A 952(1–2):13–27. doi:10.1016/S0021-9673(02)00057-2
Montesinos-Cisneros RM, Olivas Jde L, Ortega J, Guzman R, Tejeda-Mansir A (2007) Breakthrough performance of plasmid DNA on ion-exchange membrane columns. Biotechnol Prog 23(4):881–887. doi:10.1021/bp070054d
Guerrero-German P, Prazeres DM, Guzman R, Montesinos-Cisneros RM, Tejeda-Mansir A (2009) Purification of plasmid DNA using tangential flow filtration and tandem anion-exchange membrane chromatography. Bioprocess Biosyst Eng 32(5):615–623. doi:10.1007/s00449-008-0284-7
Gustavsson P-E, Larsson P-O (1996) Superporous agarose, a new material for chromatography. J Chromatogr A 734(2):231–240. doi:10.1016/0021-9673(95)01304-0
Theodossiou I, Søndergaard M, Thomas ORT (2001) Design of expanded bed supports for the recovery of plasmid DNA by anion exchange adsorption. Bioseparation 10(1):31–44. doi:10.1023/a:1012078605874
Pérez-Martínez Y, Montesinos-Cisneros RM, Guerrero-Germán P, Guzman-Zamudio R, Tejeda-Mansir A (2015) Batch equilibrium and kinetic studies of plasmid pCI adsorption onto perfusion particles. J Liq Chromatogr Relat Technol 38(2):196–200. doi:10.1080/10826076.2014.896818
García-Rendón A, Munguía Soto R, Montesinos-Cisneros RM, Guzmán R, Tejeda Mansir A (2016) Performance analysis of exponential-fed perfusion cultures for pDNA vaccines production. J Chem Technol Biotechnol. doi:10.1002/jctb.5011
Padilla-Zamudio A, Guerrero-Germán P, Tejeda-Mansir A (2015) Plasmid DNA primary recovery from E. coli lysates by depth bed microfiltration. Bioprocess Biosyst Eng 38(6):1091–1096
Manzano I, Guerrero-German P, Montesinos-Cisneros RM, Tejeda-Mansir A (2015) Plasmid DNA pre-purification by tangential flow filtration. Biotechnol Biotechnol Equip 29(3):586–591
Bohle K, Ross A (2011) Plasmid DNA production for pharmaceutical use: role of specific growth rate and impact on process design. Biotechnol Bioeng 108(9):2099–2106. doi:10.1002/bit.23138
Sanchez-Casco M, Dumonteil E, Ortega-Lopez J (2013) Production optimisation of a DNA vaccine candidate against leishmaniasis in flask culture. Afr J Biotechnol 12(31):4874–4880. doi:10.5897/ajb12.1360
Diogo MM, Queiroz JA, Prazeres DM (2003) Assessment of purity and quantification of plasmid DNA in process solutions using high-performance hydrophobic interaction chromatography. J Chromatogr A 998(1–2):109–117
Eon-Duval A, MacDuff RH, Fisher CA, Harris MJ, Brook C (2003) Removal of RNA impurities by tangential flow filtration in an RNase-free plasmid DNA purification process. Anal Biochem 316(1):66–73
Freitas S, Canario S, Santos JA, Prazeres DM (2009) Alternatives for the intermediate recovery of plasmid DNA: performance, economic viability and environmental impact. Biotechnol J 4(2):265–278. doi:10.1002/biot.200800216
Sun B, Yu X, Yin Y, Liu X, Wu Y, Chen Y, Zhang X, Jiang C, Kong W (2013) Large-scale purification of pharmaceutical-grade plasmid DNA using tangential flow filtration and multi-step chromatography. J Biosci Bioeng 116(3):281–286. doi:10.1016/j.jbiosc.2013.03.015
Zhang SY, Krivosheyeva A, Nochumson S (2003) Large-scale capture and partial purification of plasmid DNA using anion-exchange membrane capsules. Biotechnol Appl Biochem 37:245–249. doi:10.1042/Ba20030009
Acknowledgements
This research was supported by the grant provided by the National Council for Science and Technology (CONACYT) under the projects CB2012-179779 and CB2015-257411. We also appreciate the support given by the Strengthening Program Quality in Educative Institutions (PROFOCIE) 2015 and the University of Sonora.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franco-Medrano, D.I., Guerrero-Germán, P., Montesinos-Cisneros, R.M. et al. Plasmid pVAX1-NH36 purification by membrane and bead perfusion chromatography. Bioprocess Biosyst Eng 40, 463–471 (2017). https://doi.org/10.1007/s00449-016-1714-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1714-6