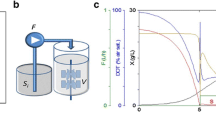
Abstract
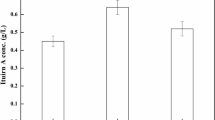
Defined and semi-defined medium-based feeding strategies were developed to enhance recombinant human growth hormone (rhGH) production by Bacillus subtilis BGSC-1A178 (scoC −) strain carrying pMK4::pre(subC)::hGH. Defined medium-based feeding strategies were designed by exponential feeding of glucose and (NH4)2HPO4 at two pre-determined specific growth rates, µ 0 = 0.10 and 0.17 h−1. Semi-defined medium-based feeding strategies were designed by exponential feeding of substrate solution consisting of glucose, (NH4)2HPO4, peptone, and trace salt solution (PTM1) at three pre-determined specific growth rates, µ 0 = 0.10, 0.17, and 0.25 h−1. At all the strategies applied, transition cultivation time from batch to fed-batch operation was t T = 4 h. The highest rhGH concentration was obtained as C rhGH = 0.5 g L−1 with semi-defined medium-based feeding strategy designed with µ 0 = 0.25 h−1 using feed substrate stock solution containing 200 g L−1 glucose, 117 g L−1 (NH4)2HPO4, 100 g L−1 peptone, and 5 mL L−1 PTM1 at t = 22 h when the cell concentration reached to C X = 8.29 g L−1. The overall product and cell yields on glucose were obtained as \(\bar{Y}_{P/S}\) = 7.21 mg g−1 and \(\bar{Y}_{X/S}\) = 0.12 g g−1, respectively. The results indicate the requirement of designing continuous feed stream in fed-batch production to enhance rhGH production by r-B. subtilis.






Similar content being viewed by others
References
Özdamar TH, Şentürk B, Yılmaz ÖD, Çalık G, Çelik E, Çalık P (2009) Expression system for recombinant human growth hormone production from Bacillus subtilis. Biotechnol Prog 25(1):75–84
Goeddel DV, Heyneker HL, Hozumi T, Arentzen R, Itakura K, Yansura DG, Ross MJ, Miozzari G, Crea R, Seeburg PH (1979) Direct expression in Escherichia coli of a DNA sequence coding for human growth hormone. Nature 281:544–548
Becker GW, Hsiung HM (1986) Expression, secretion and folding of human growth hormone in Escherichia coli. FEBS Lett 3955 204(1):145–150
Jensen EB, Carlsen S (1990) Production of recombinant human growth hormone in Escherichia coli: expression of different precursors and physiological effects on glucose, acetate, and salts. Biotechnol Bioeng 36:1–11
Nakayama A, Ando K, Kawamura K, Mita I, Fukazawa K, Hori M, Honjo M, Frutani Y (1988) Secretion of the authentic mature human growth hormone by Bacillus subtilis. J Biotechnol 8:123–134
Franchi E, Maisano F, Testori SA, Galli G, Toma S, Parente L, Ferra F, Grandi G (1991) A new human growth hormone production process using a recombinant Bacillus subtilis strain. J Biotechnol 18:41–54
Kajino T, Saito Y, Asami O, Yamada Y, Hirai M, Udata S (1997) Extracellular production of an intact and biologically active human growth hormone by the Bacillus brevis system. J Ind Microbiol Biotechnol 19:227–231
Çalık P, Orman MA, Çelik E, Halloran M, Çalık G, Özdamar TH (2008) Expression system for synthesis and purification of recombinant human growth hormone in Pichia pastoris and structural analysis by MALDI-ToF mass spectrometry. Biotechnol Prog 24:221–226
Orman MA, Çalık P, Özdamar TH (2009) The influence of carbon sources on recombinant human-growth-hormone production by Pichia pastoris is dependent on phenotype: a comparison of MutS and Mut+. Biotechnol Appl Biochem 52(3):245–255
Çalık P, İnankur B, Soyaslan EŞ, Şahin M, Taşpınar H, Bayraktar E, Açık E (2010) Fermentation and oxygen transfer characteristics in recombinant human growth hormone production by Pichia pastoris in sorbitol batch and methanol fed-batch operation. J Chem Technol Biotechnol 85:226–233
Çalık P, Bayraktar E, İnankur B, Soyaslan EŞ, Şahin M, Taşpınar H, Açık E, Yılmaz R, Özdamar TH (2010) Influence of pH on recombinant human growth hormone production by Pichia pastoris. J Chem Technol Biotechnol 85:1628–1635
Park YS, Kai K, lijima S, Kobayashi T (1992) Enhanced β-galactosidase production by high cell-density culture of recombinant Bacillus subtilis with glucose concentration control. Biotechnol Bioeng 40:686–696
Martinez A, Ramirez OT, Valle F (1998) Effect of growth rate on the production of β-galactosidase from Escherichia coli in Bacillus subtilis using glucose-limited exponentially fedbatch cultures. Enzyme Microb Technol 22:520–526
Kerovuo J, Weymarn N, Povelainen M, Auer S, Miasnikov A (2000) A new efficient expression system for Bacillus and its application to production of recombinant phytase. Biotechnol Lett 22:1311–1317
Kim DH, Oh BC, Choi WC, Lee JK, Oh TK (1999) Enzyme evaluation of Bacillus amyloliquefaciens phytase as a feed additive. Biotechnol Lett 21:925–927
Vuolanto A, Weymarn N, Kerovuo J, Ojamo H, Leisola M (2001) Phytase production by high cell density culture of recombinant Bacillus subtilis. Biotechnol Lett 23:761–766
Oh JS, Kim B, Park TH (2002) Importance of specific growth rate for subtilisin expression in fed-batch cultivation of Bacillus subtilis spoIIG mutant. Enzyme Microb Tech 30:747–751
Christiansen T, Michaelsen S, Wümpelmann M, Nielsen J (2003) Production of savinase and population viability of Bacillus clausii during high-cell-density fed-batch cultivations. Biotechnol Bioeng 83(3):344–352
Wu Q, Chen T, Gan Y, Chen X, Zhao X (2007) Optimization of riboflavin production by recombinant Bacillus subtilis RH44 using statistical designs. Appl Microb Biotechnol 76:783–794
Cho YH, Song JY, Kim KM, Kim MK, Lee IY, Kim SB, Kim HS, Han NS, Lee BH, Kim BS (2010) Production of nattokinase by batch and fed-batch culture of Bacillus subtilis. N Biotechnol 27:4
Kwon EY, Kim KM, Kim MK, Lee IY, Kim BS (2011) Production of nattokinase by high cell density fed-batch culture of Bacillus subtilis. Bioproc Biosyst Eng 34:789–793
Sibirny AA, Ubiyvovk VM, Gonchar MV, Titorenko VI, Voronovsky AY, Kapultsevich YG, Bliznik KM (1990) Reaction of direct formaldehyde oxidation to CO2 is not essential for energy supply of yeast methylotrophic growth. Arch Microbiol 154:566–575
Yamanè T, Shimizu S (1984) Fed-batch techniques in microbial processes. In: Fiechter A (ed) Biotechnology bioprocess parameters control (Book Series: Advances in Biochemical Engineering), vol 30. Springer, Berlin-Heidelberg
Çalık P, Çalık G, Özdamar TH (2000) Oxygen-transfer strategy and its regulation effects in serine alkaline protease production by Bacillus licheniformis. Biotechnol Bioeng 69(3):301–311
İleri N, Çalık P (2006) Effects of pH strategy on endo- and exo- metabolome profiles and sodium potassium hydrogen ports of beta-lactamase producing Bacillus licheniformis. Biotechnol Prog 22:411–419
Çalık P, Çalık G, Özdamar TH (1998) Oxygen transfer effects in serine alkaline protease fermentation by Bacillus licheniformis: use of citric acid as the carbon source. Enzyme Microb Technol 23:451–461
Bandyopadhyay B, Humprey AE (1967) Dynamic measurement of the volumetric oxygen transfer coefficient in fermentation systems. Biotechnol Bioeng 9:533–544
Shang L, Tian P, Kim N, Chang H, Hahm MS (2009) Effects of oxygen supply modes on the production of human growth hormone in different scale bioreactors. Chem Eng Technol 32(4):600–605
Güneş H, Boy E, Ata Ö, Zerze GH, Çalık P, Özdamar TH (2015) Methanol feeding strategy design enhances recombinant human growth hormone production by Pichia pastoris. J Chem Technol Biotechnol. doi:10.1002/jctb.4619
Acknowledgments
This work was supported by the Scientific and Technological Research Council of Turkey (TÜBİTAK) through the project 109R025 and by Middle East Technical University (METU) research fund. S.Ö. was awarded a PhD scholarship by Scientific and Technical Research Council of Turkey (TÜBİTAK). Bacillus Genetic Stock Center (BGSC) is gratefully acknowledged for providing Bacillus subtilis BGSC-1A178 (scoC −) strain.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
B. Şahin and S. Öztürk contributed equally to this work.
Rights and permissions
About this article
Cite this article
Şahin, B., Öztürk, S., Çalık, P. et al. Feeding strategy design for recombinant human growth hormone production by Bacillus subtilis . Bioprocess Biosyst Eng 38, 1855–1865 (2015). https://doi.org/10.1007/s00449-015-1426-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1426-3