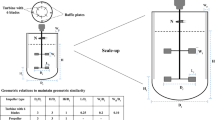
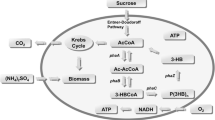
Abstract
This work presents a general model-based methodology to scale-up fed-batch bioprocesses. The idea behind this approach is to establish a dynamics hierarchy, based on a model of the process, that allows the designer to determine the proper scale factors as well as at which point of the fed-batch the process should be scaled up. Here, concepts and tools of linear control theory, such as the singular value decomposition of the Hankel matrix, are exploited in the context of process design. The proposed scale-up methodology is first described in a bioprocesses general framework highlighting its main features, key variables and parameters. Then, it is applied to a polyhydroxybutyrate (PHB) fed-batch bioreactor and compared with three empirical criteria, that are traditionally employed to determine the scale factors of these processes, showing the usefulness and distinctive features of this proposal. Moreover, this methodology provides theoretical support to a frequently used empirical rule: scale-up aerobic bioreactors at constant volumetric oxygen transfer coefficient. Finally, similar process dynamic behavior and PHB production set at the laboratory scale are predicted at the new operating scale, while it is also determined that is rarely possible to reproduce similar dynamic behavior of the bioreactor using empirical scale-up criteria.






Similar content being viewed by others
Abbreviations
- \(A_x\) :
-
Cross-sectional flow area \(\left({\rm m}^2\right)\)
- \(a_1,a_2, a_3\) :
-
Empirical coefficients for \(P_{g}\) calculation \(\left({\rm dimensionless}\right)\)
- \(c_1, c_2, c_3\) :
-
Empirical coefficients for \(K_{\rm L}{a}\) calculation \(\left({\rm dimensionless}\right)\)
- \(D_{i}\) :
-
Impeller diameter \(\left({\rm m}\right)\)
- \(f_{\rm P}\) :
-
PHB to active biomass ratio \(\left({\rm dimensionless}\right)\)
- \(f_{\rm P,m}\) :
-
PHB to active biomass ratio \(\left({\rm dimensionless}\right)\)
- \(F_{\rm N}\) :
-
Nitrogen flow rate \(\left(\frac{\rm L}{\rm h}\right)\)
- \(F_{\rm O}\) :
-
Air flow rate \(\left(\frac{\rm L}{\rm h}\right)\)
- \(F_{{\rm O}_{\rm Bias}}\) :
-
Nominal air flow rate \(\left(\frac{\rm L}{\rm h}\right)\)
- \(F_{\rm O,m}\) :
-
Maximum air flow rate \(\left(\frac{\rm L}{\rm h}\right)\)
- \(F_{\rm S}\) :
-
Substrate flow rate \(\left(\frac{\rm L}{\rm h}\right)\)
- \(k_{\rm P}\) :
-
Controller proportional gain \(\left( \frac{\rm L}{\rm gs}\right)\)
- \(K_{i{\rm N}}\) :
-
Nitrogen inhibition constant for growth \(\left( \frac{\rm g}{\rm L}\right)\)
- \(K_{i{\rm S}}\) :
-
Substrate inhibition constant for growth \(\left( \frac{\rm g}{\rm L}\right)\)
- \(K_{i{\rm PN}}\) :
-
Nitrogen inhibition constant for PHB production \(\left( \frac{\rm g}{\rm L}\right)\)
- \(K_{i{\rm PS}}\) :
-
Substrate inhibition constant for PHB production \(\left( \frac{\rm g}{\rm L}\right)\)
- \(K_{{\rm L}}a\) :
-
Volumetric oxygen transfer coefficient \(\left( \frac{1}{\rm h}\right)\)
- \(K_{\rm N}\) :
-
Saturation constant for nitrogen in growth \(\left( \frac{\rm g}{\rm L}\right)\)
- \(K_{\rm O}\) :
-
Saturation constant for oxygen in growth \(\left( \frac{\rm g}{\rm L}\right)\)
- \(K_{\rm P}\) :
-
Saturation constant for PHB in growth \(\left( {\rm dimensionless}\right)\)
- \(K_{\rm PS}\) :
-
Saturation constant for substrate in PHB production \(\left( \frac{\rm g}{\rm L}\right)\)
- \(K_{\rm S}\) :
-
Saturation constant for substrate in growth \(\left( \frac{\rm g}{\rm L}\right)\)
- \(m_{\rm O}\) :
-
Specific oxygen consumption for maintenance \(\left( \frac{1}{\rm h}\right)\)
- \(m_{\rm S}\) :
-
Specific glucose consumption for maintenance \(\left( \frac{1}{\rm h}\right)\)
- \(\mu _{\rm PS}\) :
-
Specific PHB production rate \(\left( \frac{1}{\rm h}\right)\)
- \(\mu _{\rm PS}^{\rm max}\) :
-
Maximum specific PHB production rate over substrate \(\left( \frac{1}{\rm h}\right)\)
- \(\mu _{\rm XP}\) :
-
Specific biomass growth rate over PHB \(\left( \frac{1}{\rm h}\right)\)
- \(\mu _{\rm XP}^{\rm max}\) :
-
Maximum specific biomass growth rate over PHB \(\left( \frac{1}{\rm h}\right)\)
- \(\mu _{\rm XS}\) :
-
Specific biomass growth rate over substrate \(\left( \frac{1}{\rm h}\right)\)
- \(\mu _{\rm XS}^{\rm max}\) :
-
Maximum specific biomass growth rate over substrate \(\left( \frac{1}{\rm h}\right)\)
- \(N\) :
-
Nitrogen concentration in culture broth \(\left( \frac{\rm g}{\rm L}\right)\)
- \(N_{i}\) :
-
Impeller speed \(\left( {\rm rpm}\right)\)
- \(N_{\rm F}\) :
-
Nitrogen concentration in feeding solution \(\left( \frac{\rm g}{\rm L}\right)\)
- \(N_{\rm p}\) :
-
Power number \(\left( {\rm dimensionless}\right)\)
- \({O}_{\rm G}\) :
-
Oxygen concentration in the gaseous phase \(\left( \frac{\rm g}{\rm L}\right)\)
- \({O}_{{\rm G,F}}\) :
-
Oxygen concentration in the feeding stream \(\left( \frac{\rm g}{\rm L}\right)\)
- \({O}_{\rm L}\) :
-
Dissolved oxygen concentration \(\left( \frac{\rm g}{\rm L}\right)\)
- \({O}_{\rm L,sp}\) :
-
Dissolved oxygen concentration set point \(\left( \frac{\rm g}{\rm L}\right)\)
- \({O}_{\rm L}^{\star }\) :
-
Saturation concentration of dissolved oxygen \(\left( \frac{\rm g}{\rm L}\right)\)
- \({\rm OTR}\) :
-
Oxygen transfer rate \(\left( \frac{\rm g}{\rm Lh}\right)\)
- \({P}\) :
-
PHB concentration \(\left( \frac{\rm g}{\rm L}\right)\)
- \({P}_{\rm g}\) :
-
Aerated input power \(\left( {\rm W}\right)\)
- \(r_{\rm N}\) :
-
Rate of reaction of nitrogen \(\left( \frac{\rm g}{\rm Lh}\right)\)
- \(r_{\rm O}\) :
-
Rate of reaction of oxygen \(\left( \frac{\rm g}{\rm Lh}\right)\)
- \(r_{\rm P}\) :
-
Rate of reaction of PHB \(\left( \frac{\rm g}{\rm Lh}\right)\)
- \(r_{\rm S}\) :
-
Rate of reaction of substrate \(\left( \frac{\rm g}{\rm Lh}\right)\)
- \(r_{\rm X}\) :
-
Rate of reaction of active biomass \(\left( \frac{\rm g}{\rm Lh}\right)\)
- \({S}\) :
-
Substrate concentration in culture broth \(\left( \frac{\rm g}{\rm L}\right)\)
- \({S}_{\rm F}\) :
-
Substrate concentration in feeding solution \(\left( \frac{\rm g}{\rm L}\right)\)
- \({T}\) :
-
Inner diameter of the reactor \(\left( {\rm m}\right)\)
- \(t_{\rm D}\) :
-
Derivative time for controller gain \(\left( {\rm s}\right)\)
- \(t_{\rm I}\) :
-
Integral time for controller gain \(\left( {\rm s}\right)\)
- \(V_{\rm G}\) :
-
Gas volume \(\left( {\rm L}\right)\)
- \(V_{\rm L}\) :
-
Liquid volume \(\left( {\rm L}\right)\)
- \(V_{{\rm t}}\) :
-
Impeller tip speed \(\left( \frac{\rm m}{\rm s}\right)\)
- \(V_{\rm T}\) :
-
Total fermenter volume \(\left( {\rm L}\right)\)
- \(V_{\rm L,0}\) :
-
Initial fermenter volume \(\left( {\rm L}\right)\)
- \(X\) :
-
Active biomass concentration \(\left( \frac{\rm g}{\rm L}\right)\)
- \(X_{\rm m}\) :
-
Maximum residual cell concentration \(\left( \frac{\rm g}{\rm L}\right)\)
- \(X_{\rm t}\) :
-
Total biomass concentration (\({\rm P}+{\rm X}\)) \(\left( \frac{\rm g}{\rm L}\right)\)
- \(X_{\rm t}^{\rm phase}\) :
-
Total biomass concentration (\({\rm P}+{\rm X}\)) for changing the phase \(\left( \frac{\rm g}{\rm L}\right)\)
- \(Y_{\rm PO}\) :
-
PHB yield over oxygen \(\left( {\rm dimensionless}\right)\)
- \(Y_{\rm PS}\) :
-
PHB yield over substrate \(\left( {\rm dimensionless}\right)\)
- \(Y_{\rm XN}\) :
-
Biomass yield over nitrogen \(\left( {\rm dimensionless}\right)\)
- \(Y_{\rm XO}\) :
-
Biomass yield over oxygen \(\left( {\rm dimensionless}\right)\)
- \(Y_{\rm XP}\) :
-
Biomass yield over PHB \(\left( {\rm dimensionless}\right)\)
- \(Y_{\rm XS}\) :
-
Biomass yield over substrate \(\left( {\rm dimensionless}\right)\)
- \(Z\) :
-
Liquid reactor height \(\left( {\rm dimensionless}\right)\)
- \(\alpha\) :
-
Cell density inhibition coefficient \(\left( {\rm dimensionless}\right)\)
- \(\beta\) :
-
PHB saturation power coefficient \(\left( {\rm dimensionless}\right)\)
- \(\rho _{\rm FN}\) :
-
Density of the nitrogen source stream \(\left( \frac{\rm g}{\rm L}\right)\)
- \(\rho _{\rm FS}\) :
-
Density of the substrate stream \(\left( \frac{\rm g}{\rm L}\right)\)
- \(\rho _{\rm W}\) :
-
Water density \(\left( \frac{\rm g}{\rm L}\right)\)
- \(\mu _{\rm W}\) :
-
Water viscosity \(\left( {\rm Pa\;s}\right)\)
References
Albertos P, Sala A (2004) Multivariable control systems: an engineering approach. Springer, London
Alvarez LA, Espinosa J (2012) Methodology based on SVD for control structure design. Latin Am Appl Res 42(3):245–250
Antoulas AC (2005) An overview of approximation methods for large-scale dynamical systems. Annu Rev Control 29(2):181–190. doi:10.1016/j.arcontrol.2005.08.002
Bashiri H, Heniche M, Bertrand F, Chaouki J (2014) Compartmental modelling of turbulent fluid flow for the scale-up of stirred tanks. Can J Chem Eng 92(6):1070–1081. doi:10.1002/cjce.21955
Bisio A, Kabel RL (1985) Scaleup of chemical processes conversion from laboratory scale tests to successful commercial size design. John Wiley & Sons Inc, New York
Cavaillé L, Grousseau E, Pocquet M, Lepeuple AS, Uribelarrea JL, Hernandez-Raquet G, Paul E (2013) Polyhydroxybutyrate production by direct use of waste activated sludge in phosphorus-limited fed-batch culture. Bioresour Technol 149:301–309. doi:10.1016/j.biortech.2013.09.044
Charles M (1985) Fermentation scale-up: problems and possibilities. Trends Biotechnol 3(6):134–139. doi:10.1016/0167-7799(85)90101-5
Dang NDP, Karrer DA, Dunn IJ (1977) Oxygen transfer coefficients by dynamic model moment analysis. Biotechnol Bioeng 19(6):853–865. doi:10.1002/bit.260190606
JaML Dias, Lemos PC, Serafim LS, Oliveira C, Eiroa M, Albuquerque MGE, Ramos AM, Oliveira R, Reis MAM (2006) Recent advances in polyhydroxyalkanoate production by mixed aerobic cultures: from the substrate to the final product. Macromol Biosci 6(11):885–906. doi:10.1002/mabi.200600112
Díaz-Barrera A, Gutierrez J, Martínez F, Altamirano C (2014) Production of alginate by Azotobacter vinelandii grown at two bioreactor scales under oxygen-limited conditions. Bioprocess Biosyst Eng 37(6):1133–1140. doi:10.1007/s00449-013-1084-2
Dochain D (2008) Bioprocess control. Wiley-ISTE, London
Doran PM (2013) Bioprocess engineering principles, 2nd edn. Academic Press, London
Facco P, Tomba E, Bezzo F, García-Muñoz S, Barolo M (2012) Transfer of process monitoring models between different plants using latent variable techniques. Ind Eng Chem Res 51(21):7327–7339. doi:10.1021/ie202974u
Facco P, Largoni M, Tomba E, Bezzo F, Barolo M (2014) Transfer of process monitoring models between plants: Batch systems. Chem Eng Res Des 92(2):273–284. doi:10.1016/j.cherd.2013.07.010
Fujimoto K, Scherpen JMA (2005) Nonlinear Input-normal realizations based on the differential eigenstructure of Hankel operators. IEEE Trans Autom Controli 50(1):2–18. doi:10.1109/TAC.2004.840476(410)50
Gahlawat G, Srivastava AK (2013) Development of a mathematical model for the growth associated polyhydroxybutyrate fermentation by Azohydromonas australica and its use for the design of fed-batch cultivation strategies. Bioresour Technol 137:98–105. doi:10.1016/j.biortech.2013.03.023
Garcia-Ochoa F, Gomez E (2009) Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol Adv 27(2):153–176. doi:10.1016/j.biotechadv.2008.10.006
Gasparini G, Archer I, Jones E, Ashe R (2012) Scaling up biocatalysis reactions in flow reactors. Org Process Res Dev 16(5):1013–1016
Grousseau E, Blanchet E, Déléris S, Albuquerque MGE, Paul E, Uribelarrea JL (2013) Impact of sustaining a controlled residual growth on polyhydroxybutyrate yield and production kinetics in Cupriavidus necator. Bioresour Technol 148:30–38. doi:10.1016/j.biortech.2013.08.120
Hsu YL, Wu WT (2002) A novel approach for scaling-up a fermentation system. Biochem Eng J 11(2–3):123–130. doi:10.1016/S1369-703X(02)00016-5
Hubbard DW (1987) Scaleup strategies for bioreactors containing non-newtonian broths. Ann NY Acad Sci 506(1):600–606. doi:10.1111/j.1749-6632.1987.tb23854.x
Hubbard DW, Ledger SE, Hoffman JA (1994) Scaling-up aerobic fermentation which produce non-newtonian, viscoelastic broths. In: Galindo E, Ramírez O (eds) Advances in bioprocess engineering SE-13. Springer, Netherlands, pp 95–101. doi: 10.1007/978-94-017-0641-4_13
Inglezakis VJ, Poulopoulos SG (2006) 6-Reactors scale-up. In: Adsorption, Ion exchange and catalysis design of operations and environmental applications. Elsevier, Amsterdam, pp 523–550. doi:10.1016/B978-044452783-7/50006-9
Ju LK, Chase GG (1992) Improved scale-up strategies of bioreactors. Bioprocess Eng 8(1–2):49–53. doi:10.1007/BF00369263
Majji M, Juang JN, Junkins JL (2010) Time-varying eigensystem realization algorithm. J Guid Control Dyn 33(1):13–28. doi:10.2514/1.45722
Marques MPC, Cabral JMS, Fernandes P (2010) Bioprocess scale-up: quest for the parameters to be used as criterion to move from microreactors to lab-scale. J Chem Technol Biotechnol 85(9):1184–1198. doi:10.1002/jctb.2387
Michel BJ, Miller SA (1962) Power requirements of gas-liquid agitated systems. AICHE J 8(2):262–266. doi:10.1002/aic.690080226
Monsalve-Bravo GM (2014) An approximation to the scale-up of batch processes using phenomenological-based models. Master thesis, Universidad Nacional de Colombia, Medellín
Monsalve-Bravo GM, Moscoso-Vasquez HM, Alvarez H (2014a) Scale-up of continuous reactors using phenomenological-based models. Chim Oggi-Chem Today 32(3):20–26
Monsalve-Bravo GM, Moscoso-Vasquez HM, Alvarez H (2014b) Scaleup of batch reactors using phenomenological-based models. Ind Eng Chem Res 53(22):9439–9453. doi:10.1021/ie500587r
Moucha T, Linek V, Prokopová E (2003) Gas hold-up, mixing time and gas-liquid volumetric mass transfer coefficient of various multiple-impeller configurations: rushton turbine, pitched blade and techmix impeller and their combinations. Chem Eng Sci 58(9):1839–1846. doi:10.1016/S0009-2509(02)00682-6
Mozumder MSI, De Wever H, Volcke EIP, Garcia-Gonzalez L (2014a) A robust fed-batch feeding strategy independent of the carbon source for optimal polyhydroxybutyrate production. Process Biochem 49(3):365–373. doi:10.1016/j.procbio.2013.12.004
Mozumder MSI, Goormachtigh L, Garcia-Gonzalez L, De Wever H, Volcke EIP (2014b) Modeling pure culture heterotrophic production of polyhydroxybutyrate (PHB). Bioresour Technol 155:272–280. doi:10.1016/j.biortech.2013.12.103
Nasir H, Stanković V, Marshall S (2012) Singular value decomposition based fusion for super-resolution image reconstruction. Signal Process-Image Commun 27(2):180–191. doi:10.1016/j.image.2011.12.002
Nikakhtari H, Song W, Nemati M, Hill GA (2014) Oxygen mass transfer and scale-up studies in baffled roller bioreactors. Bioprocess Biosyst Eng 37(2):193–203. doi:10.1007/s00449-013-0985-4
Oosterhuis N (1984) Chapter 1: Introduction. In: Scale-up of bioreactors: a scale-down approach. Technische Hogeschool Delft, pp 13–31
Pollard DJ, Kirschner TF, Hunt GR, Tong IT, Stieber R, Salmon PM (2007) Scale-up of a viscous fungal fermentation: application of scale-up criteria with regime analysis and operating boundary conditions. Biotechnol Bioeng 96(2):307–317. doi:10.1002/bit.21112
Ruiz AA, Alvarez H (2011) Scale-up of chemical and biochemical processes using a phenomenological model (in Spanish). Inf Tecnol 22(6):33–52. doi:10.4067/S0718-07642011000600005 (in Spanish)
Ruzicka MC (2008) On dimensionless numbers. Chem Eng Res Des 86(8):835–868. doi:10.1016/j.cherd.2008.03.007
Saa P, Moenne M, Pérez-Correa J, Agosin E (2012) Modeling oxygen dissolution and biological uptake during pulse oxygen additions in oenological fermentations. Bioprocess Biosyst Eng 35(7):1167–1178. doi:10.1007/s00449-012-0703-7
Schmidt FR (2005) Optimization and scale up of industrial fermentation processes. Appl Microbiol Biotechnol 68(4):425–435. doi:10.1007/s00253-005-0003-0
Siegel RS, Ollis DF (1984) Kinetics of growth of the hydrogen-oxidizing bacterium Alcaligenes eutrophus (ATCC 17707) in chemostat culture. Biotechnol Bioeng 26(7):764–770. doi:10.1002/bit.260260721
Simpson R, Sastry S (2013) Scale-up in chemical and bioprocess engineering. In: Chemical and bioprocess engineering SE-10. Springer New York, pp 261–275. doi:10.1007/978-1-4614-9126-2_10
Skogestad S, Postlethwaite I (2007) Multivariable feedback control: analysis and design, vol 2. John Wiley & Sons Inc, New York
Speight JG (2005) Lange’s handbook of chemistry, 16th edn, vol 1. McGraw-Hill, New York
Sweere APJ, Luyben KCAM, Kossen NWF (1987) Regime analysis and scale-down: tools to investigate the performance of bioreactors. Enzyme Microb Technol 9(7):386–398. doi:10.1016/0141-0229(87)90133-5
Takors R (2012) Scale-up of microbial processes: impacts, tools and open questions. J Biotechnol 160(1–2):3–9. doi:10.1016/j.jbiotec.2011.12.010
Third KA, Newland M, Cord-Ruwisch R (2003) The effect of dissolved oxygen on PHB accumulation in activated sludge cultures. Biotechnol Bioeng 82(2):238–250. doi:10.1002/bit.10564
Tomba E, Facco P, Bezzo F, García-Muñoz S, Barolo M (2012) Combining fundamental knowledge and latent variable techniques to transfer process monitoring models between plants. Chemometrics Intell Lab Syst 116:67–77. doi:10.1016/j.chemolab.2012.04.016
Villadsen J, Nielsen J, Lidén G (2011) Scale-up of bioprocesses. In: Bioreaction Engineering Principles SE-11. Springer, US, pp 497–546. doi:10.1007/978-1-4419-9688-6_11
van de Wal M, de Jager B (2001) A review of methods for input/output selection. Automatica 37(4):487–510. doi:10.1016/S0005-1098(00)00181-3
Wang G, Chu J, Noorman H, Xia J, Tang W, Zhuang Y, Zhang S (2014) Prelude to rational scale-up of penicillin production: a scale-down study. Appl Microbiol Biotechnol 98(6):2359–2369. doi:10.1007/s00253-013-5497-2
Xie LB, Shieh LS, Tsai JSH, Zhang Y (2013) Approximated modeling and minimal realization of transfer function matrices with multiple time delays. J Process Control 23(1):3–11. doi:10.1016/j.jprocont.2012.10.008
Xing Z, Kenty BM, Li ZJ, Lee SS (2009) Scale-up analysis for a CHO cell culture process in large-scale bioreactors. Biotechnol Bioeng 103(4):733–746. doi:10.1002/bit.22287
Yang JD, Lu C, Stasny B, Henley J, Guinto W, Gonzalez C, Gleason J, Fung M, Collopy B, Benjamino M, Gangi J, Hanson M, Ille E (2007) Fed-batch bioreactor process scale-up from 3-L to 2,500-L scale for monoclonal antibody production from cell culture. Biotechnol Bioeng 98(1):141–154. doi:10.1002/bit.21413
Yin H, Zhu Z, Ding F (2011) Model order determination using the Hankel matrix of impulse responses. Appl Math Lett 24(5):797–802. doi:10.1016/j.aml.2010.12.046
Zandvliet MJ, Doren JFM, Bosgra OH, Jansen JD, Hof PMJ (2008) Controllability, observability and identifiability in single-phase porous media flow. Comput Geosci 12(4):605–622. doi:10.1007/s10596-008-9100-3
Acknowledgments
This work was supported in part by ANPCyT (PICT 2011-0888 and PICT 2012-0037), CONICET (PIP 112-2011-01-0361), UNLP (Project 11/I164) of Argentina and Red de Macrouniversidades Públicas de America Latina y el Caribe through the program “Movilidad en el Posgrado de la RED MACRO”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monsalve-Bravo, G.M., Garelli, F., Mozumder, M.S.I. et al. Model-based scale-up methodology for aerobic fed-batch bioprocesses: application to polyhydroxybutyrate (PHB) production. Bioprocess Biosyst Eng 38, 1179–1190 (2015). https://doi.org/10.1007/s00449-015-1360-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1360-4