Abstract
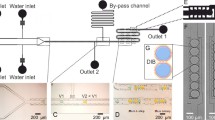
An artificially created lipid bilayer is an important platform in studying ion channels and engineered biosensor applications. However, a lipid bilayer created using conventional techniques is fragile and short-lived, and the measurement of ion channels requires expertise and laborious procedures, precluding practical applications. Here, we demonstrate a storable droplet lipid bilayer precursor frozen with ion channels, resulting in a droplet interface bilayer upon thawing. A small vial with an aqueous droplet in organic solution was flash frozen in −80 °C methanol immediately after an aqueous droplet was introduced into the organic solution and gravity draws the droplet down to the interface upon thawing. A lipid bilayer created along the interface using this method had giga-ohm resistance and typical specific capacitance values. The noise level of this system is favorably comparable to the conventional system. The subsequent incorporation of ion channels, alpha-hemolysin and gramicidin A, showed typical conductance values consistent with those in previous literatures. This novel system to create a lipid bilayer as a whole can be automated from its manufacture to use and indefinitely stored when frozen. As a result, ion channel measurements can be carried out in any place, increasing the accessibility of ion channel studies as well as a number of applications, such as biosensors, ion channel drug screening, and biophysical studies.




Similar content being viewed by others
Abbreviations
- HTS:
-
High-throughput screening
- hERG:
-
Human ether-á-go-go related gene
- DPhPC:
-
1,2-Diphytanoyl-sn-glycero-3-phosphocholine
References
Moller C (2010) Keeping the rhythm: hERG and beyond in cardiovascular safety pharmacology. Expert Rev Clin Pharmacol 3(3):321–329
Willumsen NJ, Bech M, Olesen SP, Jensen BS, Korsgaard MPG, Christophersen P (2003) High throughput electrophysiology: new perspectives for ion channel drug discovery. Recept Channels 9(1):3–12
Kandel ER, Schwartz JH, Jessell TM (2000) Principles of neural science, 4th edn. McGraw-Hill, Health Professions Division, New York
Wang XB, Li M (2003) Automated electrophysiology: high throughput of art. Assay Drug Dev Technol 1(5):695–708
Wood C, Williams C, Waldron GJ (2004) Patch clamping by numbers. Drug Discov Today 9(10):434–441
Hanke W, Schlue WR (1993) Planar lipid bilayers: methods and applications. Academic Press, London
Miller C (1986) Ion channel reconstitution. Plenum Press, New York
Tien HT, Ottova-Leitmannova A (2003) Planar lipid bilayers (BLMs) and their appilcations. Membrane science and technology series, vol 7, 1st edn. Elsevier, Boston
Bayley H, Cremer PS (2001) Stochastic sensors inspired by biology. Nature 413(6852):226–230
Cornell BA, Braach-Maksvytis VLB, King LG, Osman PDJ, Raguse B, Wieczorek L, Pace RJ (1997) A biosensor that uses ion-channel switches. Nature 387(6633):580–583
Jeon TJ, Malmstadt N, Schmidt JJ (2006) Hydrogel-encapsulated lipid membranes. J Am Chem Soc 128(1):42–43
Malmstadt N, Nash MA, Purnell RF, Schmidt JJ (2006) Automated formation of lipid-bilayer membranes in a microfluidic device. Nano Lett 6(9):1961–1965
Suzuki H, Tabata KV, Noji H, Takeuchi S (2006) Highly reproducible method of planar lipid bilayer reconstitution in polymethyl methacrylate microfluidic chip. Langmuir 22(4):1937–1942
Funakoshi K, Suzuki H, Takeuchi S (2006) Lipid bilayer formation by contacting monolayers in a microfluidic device for membrane protein analysis. Anal Chem 78(24):8169–8174
Maglia G, Heron AJ, Hwang WL, Holden MA, Mikhailova E, Li Q, Cheley S, Bayley H (2009) Droplet networks with incorporated protein diodes show collective properties. Nat Nanotechnol 4(7):437–440
Bayley H, Cronin B, Heron A, Holden MA, Hwang WL, Syeda R, Thompson J, Wallace M (2008) Droplet interface bilayers. Mol Biosyst 4(12):1191–1208
Ide T, Kobayashi T, Hirano M (2008) Lipid bilayers at the gel interface for single ion channel recordings. Anal Chem 80(20):7792–7795
Ide T, Takeuchi Y, Aoki T, Yanagida T (2002) Simultaneous optical and electrical recording of a single ion-channel. Jpn J Physiol 52:429–434
Thompson JR, Heron AJ, Santoso Y, Wallace MI (2007) Enhanced stability and fluidity in droplet on hydrogel bilayers for measuring membrane protein diffusion. Nano Lett 7(12):3875–3878
Stanley CE, Elvira KS, Niu XZ, Gee AD, Ces O, Edel JB, Demello AJ (2010) A microfluidic approach for high-throughput droplet interface bilayer (DIB) formation. Chem Commun 46:1620–1622
Creasy MA, Leo DJ (2010) Non-invasive measurement techniques for measuring properties of droplet interface bilayers. Smart Mater Struct 19:094016
Poulos JL, Jeon TJ, Damoiseaux R, Gillespie EJ, Bradley KA, Schmidt JJ (2009) Ion channel and toxin measurement using a high throughput lipid membrane platform. Biosens Bioelectron 24(6):1806–1810
Smith A (2002) Screening for drug discovery: the leading question. Nature 418(6896):453–459
Jeon TJ, Poulos JL, Schmidt JJ (2008) Long-term storable and shippable lipid bilayer membrane platform. Lab Chip 8(10):1742–1744
Poulos JL, Jeon TJ, Schmidt JJ (2010) Automatable production of shippable bilayer chips by pin tool deposition for an ion channel measurement platform. Biotechnol J 5(5):511–514
Carte AE (1961) Air bubbles in ice. Proc Phys Soc 77:757
Janko K, Benz R (1977) Properties of lipid bilayer membranes made from lipids containing phytanic acid. Biochim Biophys Acta (BBA) Biomembr 470(1):8–16
Bayley H (2006) Sequencing single molecules of DNA. Curr Opin Chem Biol 10(6):628–637
Stankovic CJ, Heinemann SH, Delfino JM, Sigworth FJ, Schreiber SL (1989) Transmembrane channels based on tartaric acid-gramicidin A hybrids. Science 244(4906):813–817
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (#2009-0068624, #2009-0073070) and Inha University Research Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, SH., Choi, S., Kim, YR. et al. Storable droplet interface lipid bilayers for cell-free ion channel studies. Bioprocess Biosyst Eng 35, 241–246 (2012). https://doi.org/10.1007/s00449-011-0602-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0602-3