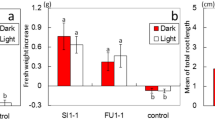
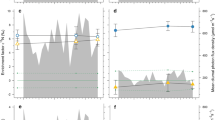
Abstract
Partially mycoheterotrophic (mixotrophic) plants gain carbon from both photosynthesis and their mycorrhizal fungi. This is considered an ancestral state in the evolution of full mycoheterotrophy, but little is known about this nutrition, and especially about the physiological balance between photosynthesis and fungal C gain. To investigate possible compensation between photosynthesis and mycoheterotrophy in the Mediterranean mixotrophic orchid Limodorum abortivum, fungal colonization was experimentally reduced in situ by fungicide treatment. We measured photosynthetic pigments of leaves, stems, and ovaries, as well as the stable C isotope compositions (a proxy for photosynthetic C gain) of seeds and the sizes of ovaries and seeds. We demonstrate that (1) in natural conditions, photosynthetic pigments are most concentrated in ovaries; (2) pigments and photosynthetic C increase in ovaries when fungal C supply is impaired, buffering C limitations and allowing the same development of ovaries and seeds as in natural conditions; and (3) responses to light of pigment and 13C contents in ovaries shift from null responses in natural conditions to responses typical of autotrophic plants in treated L. abortivum, demonstrating photoadaptation and enhanced use of light in the latter. L. abortivum thus preferentially feeds on fungi in natural conditions, but employs compensatory photosynthesis to buffer fungal C limitations and allow seed development.





Similar content being viewed by others
References
Abadie JC, Puttsepp U, Gebauer G, Faccio A, Bonfante P, Selosse MA (2006) Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and nonphotosynthetic individuals. Can J Bot 84:1462–1477
Ahmed AM, Heikal MD, Hindawy OS (1983) Side effects of benomyl (fungicide) treatments on sunflower, cotton and cowpea plants. Phyton 23(2):185–195
Anisimova M, Gascuel O (2006) Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol 55(4):539–552
Anisimova M, Gil M, Dufayard JF, Dessimoz C, Gascuel O (2011) Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst Biol 60(5):685–699
Aschan G, Pfanz H (2003) Non-foliar photosynthesis—a strategy of additional carbon acquisition. Flora 198:81–97
Bayman P, González EJ, Fumero JJ, Tremblay RL (2002) Are fungi necessary? How fungicides affect growth and survival of the orchid Lepanthes rupestris in the field. J Ecol 90:1002–1008
Beyrle HF, Smith SE (1993) Excessive carbon prevents greening of leaves in mycorrhizal seedlings of the terrestrial orchid Orchis morio. Lindleyana 8:97–99
Bidartondo MI (2005) The evolutionary ecology of myco-heterotrophy. New Phytol 167:335–352
Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ (2004) Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc R Soc B 270:835–842
Brotas V, Plante-Cuny M (2003) The use of HPLC pigment analysis to study microphytobenthos communities. Acta Oecol 24:109–115
Cameron DD, Preiss K, Gebauer G, Read DJ (2009) The chlorophyll-containing orchid Corallorhiza trifida derives little carbon through photosynthesis. New Phytol 138:358–364
Cernusak LA, Tcherkez G, Keitel C, Cornwell WK, Santiago LS, Knohl A, Barbour MM, Williams DG, ReichI PB, Ellsworth DS, Dawson TE, Griffiths HG, Farquhar GD, Wright IJ (2009) Why are non-photosynthetic tissues generally 13C enriched compared to leaves in C3 plants? Review and synthesis of current hypotheses. Funct Plant Biol 36:199–213
Diaz G, Carrillo C, Honrubia M (2003) Differential responses of ectomycorrhizal fungi to pesticides in pure culture. Cryptogam Mycol 24(3):199–211
Eberhardt U (2002) Molecular kinship analyses of the agaricoid Russulaceae: correspondence with mycorrhizal anatomy and sporocarp features in the genus Russula. Mycol Prog 1(2):201–223
Ehleringer JR, Field CB, Lin ZF, Kuo CY (1986) Leaf carbon isotope and mineral composition in subtropical plants along an irradiance cline. Oecologia 70:520–526
Farquhar GD, Ehleringer JR, Hubick TK (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Phys 40:503–537
Gebauer G, Meyer M (2003) 15N and 13C natural abundance of autotrophic and mycohetero-trophic orchids provides insight into nitrogen and carbon gain from fungal association. New Phytol 160:209–223
Girlanda M, Selosse MA, Cafasso D, Brilli F, Delfine S, Fabbian R, Ghignone S, Pinelli P, Segreto R, Loreto F, Cozzolino S, Perotto S (2006) Inefficient photosynthesis in the Mediterranean orchid Limodorum abortivum is mirrored by specific association to ectomycorrhizal Russulaceae. Mol Ecol 15:491–504
Grimmer MK, Foulkes MJ, Paveley ND (2012) Foliar pathogenesis and plant water relations: a review. J Exp Bot 63:4321–4331
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59(3):307–321
Heinonen-Tanski H, Holopainen T (1991) Maintenance of ectomycorrhizal fungi. In: Norris JR, Read DJ, Varma AK (eds) Techniques for the study of mycorrhiza. Methods in Microbiology, vol. 23. Academic, New York, pp 413–422
Hynson NA, Madsen TP, Selosse MA, Adam IKU, Ogura-Tsujita Y, Roy M, Gebauer G (2013) The physiological ecology of mycoheterotrophy (Chap. 8). In: Merckx V (ed) Mycoheterotrophy: the biology of plants living on fungi. Springer, Berlin, pp 297–342
Julou T, Burghardt B, Gebauer G, Berveiller D, Damesin C, Selosse MA (2005) Mixotrophy in orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytol 166:639–653
Kaufmann JE, Williamson DR (1981) Chemical manipulation of stomatal number and behavior in Merion Kentucky bluegrass (Poa pratensis L.). In: Sheard RW (ed) Proceedings of the 4th International Turfgrass Research Conference. Ontario Agricultural College, Guelph, pp 501–508
Klooster MR, Clark DL, Culle TM (2009) Cryptic bracts facilitate herbivore avoidance in the mycoheterotrophic plant Monotropsis odorata (Ericaceae). Am J Bot 96:1–9
Küpper H, Seibert S, Parameswaran A (2007) Fast, sensitive, and inexpensive alternative to analytical pigment HPLC: quantification of chlorophylls and carotenoids in crude extracts by fitting with Gauss Peak Spectra. Anal Chem 79:7611–7627
Leake JR (1994) The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol 127:171–216
Leake JR (2004) Myco-heterotroph/epiparasitic plant interactions with ectomycorrhizal and arbuscular mycorrhizal fungi. Curr Opin Plant Biol 7:422–428
Lichtenthaler HK, Ač A, Marek MV, Kalina J, Urban O (2007a) Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiol Biochem 45(8):577–588
Lichtenthaler HK, Babani F, Langsdorf G (2007b) Chlorophyll fluorescence imaging of photosynthetic activity in sun and shade leaves of trees. Photosynth Res 93(1–3):235–244
Martos F, Dulormne M, Pailler T, Bonfante P, Faccio A, Fournel J, Dubois MP, Selosse MA (2009) Independent recruitment of saprotrophic fungi as mycorrhizal partners by tropical achlorophyllous orchids. New Phytol 184(3):668–681
Matsuda Y, Shimizu S, Mori M, Ito SI, Selosse MA (2012) Seasonal and environmental changes of mycorrhizal associations and heterotrophy levels in mixotrophic Pyrola japonica (Ericaceae) growing under different light environments. Am J Bot 99(7):1177–1188
Merckx V, Freudenstein JV (2010) Evolution of mycoheterotrophy in plants: a phylogenetic perspective. New Phytol 185:605–609
Montfort C, Küsters G (1940) Saprophytismus und Photosynthese I. Biochemische und physiologische Studien an Humus-Orchideen. Bot Archiv 40:571–633
Paduano C, Rodda M, Ercole E, Girlanda M, Perotto S (2011) Pectin localization in the Mediterranean orchid Limodorum abortivum reveals modulation of the plant interface in response to different mycorrhizal fungi. Mycorrhiza 21(2):97–104
Podani J (2007) Analisi ed esplorazione multivariata dei dati in ecologia e biologia. Liguori Editore, Napoli
Preiss K, Adam IKU, Gebauer G (2010) Irradiance governs exploitation of fungi: fine-tuning of carbon gain by two partially myco-heterotrophic orchids. Proc R Soc B 277:1333–1336
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasmussen H (1995) Terrestrial orchids—from seed to mycotrophic plant. Cambridge University Press, Cambridge
Roy M, Gonneau C, Rocheteau A, Berveiller D, Thomas JC, Damesin C, Selosse MA (2013) Why do mixotrophic plants stay green? A comparison between green and achlorophyllous orchid individuals in situ. Ecol Monogr 83:95–117
Roy M, Watthana S, Stier A, Richard F, Vessabutr S, Selosse MA (2009) Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biol 7:51
Ruzin SE (1999) Plant microtechnique and microscopy. Oxford University Press, Oxford
Schliep KP (2011) phangorn: phylogenetic analysis in R. Bioinformatics 27(4):592–593
Selosse MA, Cameron DD (2010) Introduction to a Virtual Special Issue on mycoheterotrophy: New Phytologist sheds light on non-green plants. New Phytol 185:591–593.
Selosse MA, Roy M (2009) Green plants that feed on fungi: facts and questions about mixotrophy. Trends Plant Sci 14:64–70
Selosse MA, Weiss M, Jany JL, Tillier A (2002) Communities and populations of sebacinoid basidiomycetes associated with the achlorophyllous orchid Neottia nidus-avis (L.) LCM Rich. and neighbouring tree ectomycorrhizae. Mol Ecol 11:1831–1844
Šesták Z, Čatský J (1962) Intensity of photosynthesis and chlorophyll content as related to leaf age in Nicotiana sanderae. Hort. Biol Plant 4(2):131–140
Stöckel M, Meyer C, Gebauer G (2011) The degree of mycoheterotrophic carbon gain in green, variegated and vegetative albino individuals of Cephalanthera damasonium is related to leaf chlorophyll concentrations. New Phytol 189:790–796
Tedersoo L, Pellet P, Koljalg U, Selosse MA (2007) Parallel evolutionary paths to micoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for myxotrophy in Pyroleae. Oecologia 151:206–217
Veldre V, Abarenkov K, Bahram M, Martos F, Selosse MA, Tamm H, Koljalg U, Tedersoo L (2013) Insights into the evolution of nutritional modes of Ceratobasidiaceae (Cantharellales, Basidiomycota) as revealed from publicly available ITS sequences. Fungal Ecol 6(4):256–268
Yukari K, Naoya S, Hisayoshi Y (2014) Both live and degenerating pelotons transfer C and N to symbiotic orchid protocorms as revealed by stable isotope cellular imaging. New Phytol (in press)
Zimmer K, Meyer C, Gebauer G (2008) The ectomicorrhizal specialist orchid Corallorhiza trifida is a partial myco-heterotroph. New Phytol 178:395–400
Acknowledgments
This unfunded work was performed due to the courtesy and generosity of those who helped the authors—lending their instruments, sharing their knowledge and time—without any financial interest. To them we express our gratitude. We are infinitely grateful to Prof. Kazuhide Nara (University of Tokyo, Japan), who provided us with the plates containing Russula, and to Mr. Mario Corrado for giving us his permission to perform the field trial in his estate. We must also thank Dr. Agostino Menna and Dr. Beatrice Cocozziello (ARPA Campania, Italy) for giving us their permission to use—and their help in using—the HPLC system; Dr. Mathieu Sauve (CEFE-CNRS, France) for help with fungal barcoding; Prof. Roberto Ligrone (Seconda Università di Napoli, Italy) for his helpful review of the manuscript; and Dr. David Marsh for revising the English.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Caroline Müller.
Alessandro Bellino began this research, then Daniela Baldantoni worked alongside him in every phase of this project, from devising the experimental plan to the samplings, analyses, and interpretation of data. Marc-André Selosse proposed additional experiments and measurements, and carried out some of them in his lab. Rossella Guerrieri and Marco Borghetti performed the \(\delta ^{13}\)C measurements. Anna Alfani coordinated the work. All contributed to writing the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bellino, A., Alfani, A., Selosse, MA. et al. Nutritional regulation in mixotrophic plants: new insights from Limodorum abortivum . Oecologia 175, 875–885 (2014). https://doi.org/10.1007/s00442-014-2940-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2940-8