Abstract
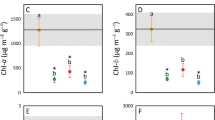
Diverse invertebrate and vertebrate species live in association with plants of the large Neotropical family Bromeliaceae. Although previous studies have assumed that debris of associated organisms improves plant nutrition, so far little evidence supports this assumption. In this study we used isotopic (15N) and physiological methods to investigate if the treefrog Scinax hayii, which uses the tank epiphytic bromeliad Vriesea bituminosa as a diurnal shelter, contributes to host plant nutrition. In the field, bromeliads with frogs had higher stable N isotopic composition (δ15N) values than those without frogs. Similar results were obtained from a controlled greenhouse experiment. Linear mixing models showed that frog feces and dead termites used to simulate insects that eventually fall inside the bromeliad tank contributed, respectively, 27.7% (±0.07 SE) and 49.6% (±0.50 SE) of the total N of V. bituminosa. Net photosynthetic rate was higher in plants that received feces and termites than in controls; however, this effect was only detected in the rainy, but not in the dry season. These results demonstrate for the first time that vertebrates contribute to bromeliad nutrition, and that this benefit is seasonally restricted. Since amphibian–bromeliad associations occur in diverse habitats in South and Central America, this mechanism for deriving nutrients may be important in bromeliad systems throughout the Neotropics.


Similar content being viewed by others
References
Anderson B, Midgley JJ (2002) It takes two to tango but three is a tangle: mutualists and cheaters on the carnivorous plant Roridula. Oecologia 132:369–373
Anderson B, Midgley JJ (2003) Digestive mutualism, an alternate pathway in plant carnivory. Oikos 102:221–224
Anderson WB, Polis GA (1999) Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California Islands. Oecologia 118:324–332
Benzing DH (2000) Bromeliaceae: profile of an adaptive radiation. Cambridge University Press, Cambridge
Benzing DH, Givnish TJ, Bermudes D (1985) Absorptive trichomes in Brocchinia reducta (Bromeliaceae) and their evolutionary and systematic significance. Syst Bot 10:81–91
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254
Cambuí CA, Mercier H (2006) Organic nitrogen nutrition in plants. Curr Top Plant Biol 7:13–17
Carvalho-e-Silva SP, Carvalho-e-Silva AMT (2004) Scinax hayii. IUCN 2008. 2008 IUCN red list of threatened species. http://www.iucnredlist.org. Downloaded 22 December 2008
Carvalho-e-Silva SP, Izecksohn E, Carvalho-e-Silva AMPT (2000) Diversidade e ecologia de anfíbios em restingas do sudeste brasileiro. In: Esteves FA, Lacerda LD (eds) Ecologia de restingas e lagoas costeiras. NUPEM/UFRJ, Macaé, Rio de Janeiro, pp 89–97
Conte CE, Rossa-Feres DC (2006) Diversidade e ocorrência temporal da anurofauna (Amphibia, Anura) em São José dos Pinhais, Paraná, Brasil. Rev Bras Zool 23:162–175
Cruz CAG, Pimenta BVS, Silvano DL (2003) Duas novas espécies pertencentes ao complexo de Hyla albosignata Lutz & Lutz, 1938, do leste do Brasil (Amphibia, Anura, Hylidae). Bol Mus Nacl Zool 503:1–13
Duellman WE, Trueb L (1994) Biology of amphibians. Johns Hopkins University Press, Baltimore
Endres L, Mercier H (2001) Ammonium and urea as nitrogen sources for bromeliads. J Plant Physiol 158:205–212
Endres L, Mercier H (2003) Amino acid uptake and profile in bromeliads with different habitats cultivated in vitro. Plant Physiol Biochem 41:181–187
Faivovich J, Haddad CFB, Garcia PCA, Frost DR, Campbell JA, Wheeler WC (2005) Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bull Am Mus Nat Hist 294:1–240
Fischer RC, Wanek W, Richter A, Mayer V (2003) Do ants feed plants? A 15N labeling study of nitrogen fluxes from ants to plants in the mutualism of Pheidole and Pipier. J Ecol 91:126–134
Giaretta AA (1996) Reproductive specializations of the bromeliad hylid frog Phyllodytes luteolus. J Herpetol 30:96–97
Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD (1984) Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. Am Nat 124:479–497
Haddad CFB, Pombal JP (1998) Redescription of Physalaemus spiniger (Anura: Leptodactylidae) and description of two new reproductive modes. J Herpetol 32:557–565
Haddad CFB, Prado CPA (2005) Reproductive modes in frogs and their unexpected diversity in the Atlantic forest of Brazil. Bioscience 55:207–217
Inselsbacher E, Cambui CA, Richter A, Stange CF, Mercier H, Wanek W (2007) Microbial activities and foliar uptake of nitrogen in the epiphytic bromeliad Vriesea gigantea. New Phytol 175:311–320
Krungel P, Richter S (1995) Syncope antenori—a bromeliad breeding frog with free-swimming, non-feeding tadpoles (Anura: Microhylidae). Copeia 1995:955–963
Laube S, Zotz G (2003) Which abiotic factors limit vegetative growth in a vascular epiphyte? Funct Ecol 17:598–604
Lehninger AL, Nelson DL, Cox MM (1993) Amino acid oxidation and the production of urea. In: Lehninger AL, Nelson DL, Cox MM (eds) Principles of biochemistry, 2nd edn. Worth, New York, pp 503–541
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Martin GE (1994) Physiological ecology of the Bromeliaceae. Bot Rev 60:1–82
Ngai JT, Srivastava DS (2006) Predators accelerate nutrient cycling in a bromeliad ecosystem. Science 314:963
Ovaska K (1991) Reproductive phenology, population structure, and habitat use of the frog Eleutherodactylus johnstonei in Barbados, West Indies. J Herpetol 25:424–430
Owen TP Jr, Thomson WW (1988) Sites of leucine, arginine, and glycine accumulation in the absorptive trichomes of a carnivorous bromeliad. J Ultrastruct Mol Struct Res 101:215–223
Peixoto OL (1995) Associação de anuros a bromeliáceas na Mata Atlântica. Rev Univ Rural Ser Ciênc Vida 17:75–83
Phillips DL, Gregg JW (2001) Uncertainty in source partitioning using stable isotopes. Oecologia 127:171–179
Purtauf T, Scheu S (2005) Nutrient and resource dynamics and food webs: understanding the mutual relationships between the dynamics of food webs, resources, and nutrients. In: Ruiter P, Wolters V, Moore JC (eds) Dynamic food webs: multispecies assemblages, ecosystem development, and environmental change. Academic Press, MA
Richardson BA (1999) The bromeliad microcosm and the assessment of faunal diversity in a neotropical forest. Biotropica 31:321–336
Rico-Gray V, Barber JT, Thien LB, Ellgaard EG, Toney JJ (1989) An unusual animal-plant interaction: feeding of Schomburgkia tibicinis (Orchidaceae) by ants. Am J Bot 76:603–608
Romero GQ (2006) Geographic range, habitats, and host plants of bromeliad-living jumping spiders (Salticidae). Biotropica 38:522–530
Romero GQ, Mazzafera P, Vasconcellos-Neto J, Trivelin PCO (2006) Bromeliad-living spiders improve host plant nutrition and growth. Ecology 87:803–808
Romero GQ, Vasconcellos-Neto J, Trivelin PCO (2008) Spatial variation in the strength of mutualism between a jumping spider and a terrestrial bromeliad: evidence from the stable isotope 15N. Acta Oecol 33:380–386
Sagers CL, Ginger SM, Evans RD (2000) Carbon and nitrogen isotopes trace nutrient exchange in an ant-plant mutualism. Oecologia 123:582–586
Sakai WS, Sanford WG (1980) Ultrastructure of the water-absorbing trichomes of pineapple (Ananas comosus, Bromeliaceae). Ann Bot 46:7–11
Schineider JAP, Teixeira RL (2001) Relacionamento entre anfíbios anuros e bromélias da restinga de Regência, Linhares, Espírito Santo, Brasil. Iheringia 91:41–48
Solano PJ, Dejean A (2004) Ant-fed plants: comparison between three geophytic myrmecophytes. Biol J Linn Soc 83:433–439
Teixeira RL, Schineider JAP, Almeida GI (2002) The occurrence of amphibians in bromeliads from a southeastern Brazilian Restinga habitat, with special reference to Aparasphenodon brunoi (Anura, Hylidae). Braz J Biol 62:263–268
Treseder KK, Davidson DW, Ehleringer JR (1995) Absorption of ant-provided carbon and nitrogen by a tropical epiphyte. Nature 357:137–139
Versieux LM, Wendt T (2007) Bromeliaceae diversity and conservation in Minas Gerais state, Brazil. Biodivers Conserv 16:2989–3009
Wait DA, Aubrey DP, Anderson WB (2005) Seabird guano influences on desert islands: soil chemistry and herbaceous species richness and productivity. J Arid Environ 60:681–695
Yang LH (2004) Periodical cicadas as resource pulses in North American forests. Science 306:1565–1567
Young AM (1979) Arboreal movement and tadpole-carrying behavior of Dendrobates pumilio Schmidt (Dendrobatidae) in northeastern Costa Rica. Biotropica 11:238–239
Acknowledgments
The authors thank J. Purcell for comments on the manuscript and language corrections, and J. C. Souza for help with data collection in the field. C. A. Cambuí and R. P. Andreoli helped with the protein and gas exchange analyses, respectively, and G. Martinelli identified the bromeliad. G. Q. Romero was supported by research grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 04/13658-5 and 05/51421-0). F. Nomura was supported by research grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; 3300415-3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Zoe Cardon.
Rights and permissions
About this article
Cite this article
Q. Romero, G., Nomura, F., Gonçalves, A.Z. et al. Nitrogen fluxes from treefrogs to tank epiphytic bromeliads: an isotopic and physiological approach. Oecologia 162, 941–949 (2010). https://doi.org/10.1007/s00442-009-1533-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-009-1533-4