Abstract
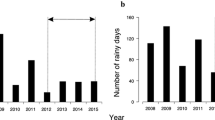
We studied the population ecology of the West African pig-nosed frog, Hemisus marmoratus, to understand the relative contributions of adult survival and recruitment to population growth rate in savannah frogs using mark-recapture modelling. We marked a total of 821 adult frogs over 6 years and recaptured 74 at least once between years. Between-year adult survival was sex-specific and varied between 0.06 and 0.53 for males and 0.07–0.41 for females. Adult survival was significantly associated with annual rainfall and is cause for concern if rainfall declines further in the study region as predicted by changes in the global climate. There was a significant interaction between rainfall and sex with dry weather having a stronger negative effect on males than females. Pig-nosed frogs experienced boom and bust years with a single decline more dramatic than increases. Recruitment (in situ and immigration; 0.67–0.88) was substantially more important than adult survival (0.12–0.33) in determining realised population growth. In situ recruitment was highly variable between years with 1–36% of eggs and tadpoles released by females into the pond surviving to metamorphosis. Years of low tadpole survival were associated with high numbers of predatory tortoises. Thus, like other pond-breeding anurans, pig-nosed frogs showed highly variable juvenile recruitment, low adult survival and density-independent effects on population growth by predators and weather.

Similar content being viewed by others
References
Alford RA, Richards SJ (1999) Global amphibian declines: a problem in applied ecology. Annu Rev Ecol Syst 30:133–165
Altwegg R, Reyer H-U (2003) Patterns of natural selection on size at metamorphosis in water frogs. Evolution 57:872–882
Anderson DR, Burnham KP (1999a) General strategies for the analysis of ringing data. Bird Study 46 [Suppl]:261–270
Anderson DR, Burnham KP (1999b) Understanding information criteria for selection among capture-recapture or ring recovery models. Bird Study 46(suppl):14–21
Anholt BR, Hotz H, Guex G-D, Semlitsch RD (2003) Overwinter survival of Rana lessonae and its hemiclonal associate Rana esculenta. Ecology 84:391–397
Biek R, Funk WC, Maxell BA, Mills LS (2002) What is missing in amphibian decline research: insights from ecological sensitivity analysis. Conserv Biol 16:728–734
Blaustein AR, Kiesecker JM, Chivers DP, Hokit DG, Marco A, Belden LK, Hatch A (1998) Effects of ultraviolet radiation on amphibians: field experiments. Am Zool 38:799–812
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theoretic approach. Springer, Berlin Heidelberg New York
Caldwell JP (1987) Demography and life history of two species of chorus frogs (Anura: Hylidae) in South Carolina. Copeia 1987:114–127
Choquet R, Reboulet AM, Pradel R, Gimenez O, Lebreton JD (2003) U-Care version 2.0. CEFE/CNRS, Montpellier. ftp://ftp.cefe.cnrs-mop.fr/biom/Soft-CR/
Conroy SDS, Brook BW (2003) Demographic sensitivity and persistence of the threatened white- and orange-bellied frogs of Western Australia. Popul Ecol 45:105–114
Cooch E, White G (2003) Using mark—a gentle introduction, 2nd edn. http://wwwcnrcolostateedu/~gwhite/mark/markhtm
Eggleton P, Bignell DE, Sands WA, Mawdsley NA, Lawton JH, Wood TG, Bignell NC (1996) The diversity, abundance and biomass of termites under differing levels of disturbance in the Mbalmayo Forest Reserve, southern Cameroon. Philos Trans R Soc Lond B 351:51–68
Gomez-Mestre I, Keller C (2003) Experimental assessment of turtle predation on larval anurans. Copeia 2003:349–356
Grafe TU, Kaminsky SK, Linsenmair KE (in press) Terrestrial larval development and nitrogen excretion in the afro-tropical pig-nosed frog, Hemisus marmoratus. J Trop Ecology
Green DM (2003) The ecology of extinction: population fluctuation and decline in amphibians. Biol Conserv 111:331–343
Hellriegel B (2000) Singe- or multistage regulation in complex life cycles: does it make a difference? Oikos 88:239–249
Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL (2000) Quantitative evidence for global amphibian population declines. Nature 404:752–755
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108
Kaminsky SK, Linsenmair KE, Grafe TU (1999) Reproductive timing, nest construction and tadpole guidance in the African pig-nosed frog Hemisus marmoratus. J Herpetol 33:119–123
Kaminsky SK, Grafe TU, Spieler M, Linsenmair KE (2004) A new method for immobilizing fossorial frogs after radio-transmitter implantation and notes on movement patterns in the pig-nosed frog, Hemisus marmoratus. Herpetol Rev 35:146–148
Kendall WL, Pollock KH, Brownie C (1995) A likelihood-based approach to capture-recapture estimation of demographic parameters under the robust design. Biometrics 51:293–308
Kiesecker JM, Blaustein AR (1998) Effects of introduced bullfrogs and smallmouth bass on microhabitat use, growth, and survival of native red-legged frogs (Rana aurora). Conserv Biol 12:776–787
Kluge AG (1981) The life history, social organization, and parental behavior of the gladiator frog Hyla rosenbergi Boulenger, a nest-building gladiator frog. Miscellaneous publications of the University of Michigan Museum of Zoology, vol 160. University of Michigan Museum of Zoology, Mich., pp 1–170
Kuhn J (1994) Lebensgeschichte und Demographie von Erdkrötenweibchen Bufo bufo bufo (L.). Z Feldherpetol 1:3–87
Lampert KP, Linsenmair KE (2002) Alternative life cycle strategies in the West African reed frog Hyperolius nitidulus: the answer to an unpredictable environment? Oecologia 130:364–372
Lebreton J-D, Burnham KP, Clobert J, Anderson DR (1992) Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol Monogr 62:67–118
Linsenmair KE (1997) Risk spreading and risk reducing tactics of West African anurans in an unpredictable and stressful environment. In: Newbery DM, Brown N, Prins HHT (eds) Dynamics of tropical communities. Blackwell, Oxford, pp 221–241
Meyer AH, Schmidt BR, Grossenbacher K (1998) Analysis of three amphibian populations with quarter-century long time-series. Proc R Soc Lond B Biol Sci 265:523–528
Murphy PJ (2003) Context-dependent reproductive site choice in a Neotropical frog. Behav Ecol 14:626–633
Nichols JD, Hepp GR, Pollock KH, Hines JE (1987) The Husting dilemma: a methodological note. Ecology 68:213–217
Nichols JD, Hines JE, Lebreton J-D, Pradel R (2000) Estimation of contributions to population growth. a reverse-time capture-recapture approach. Ecology 81:3362–3376
Pechmann JHK, Scott DE, Semlitsch RD, Caldwell JP, Vitt LJ, Gibbons JW (1991) Declining amphibian populations: the problem of separating human impacts from natural fluctuations. Science 253:892–895
Pollack KH, Nichols JD, Brownie C, Hines JE (1990) Statistical inference for capture-recapture experiments. Wildl Monogr 107:1–197
Pradel R (1996) Utilization of capture-mark-recapture for the study of recruitment and population growth rate. Biometrics 53:60–72
Richter SC, Seigel RA (2002) Annual variation in the population ecology of the endangered gopher frog, Rana sevosa goin and netting. Copeia 2002:962–972
Richter SC, Young JE, Johnson GN, Seigel RA (2003) Stochastic variation in reproductive success of a rare frog, Rana sevosa: implications for conservation and for monitoring amphibian populations. Biol Conserv 111:171–177
Riis N (1991) A field study of survival, growth, biomass and temperature dependence of Rana dalmatina and Rana temporaria larvae. Amphibia–Reptilia 12:229–243
Rödel M-O (1999) Predation on tadpoles by hatchlings of the freshwater turtle Pelomedusa subrufa. Amphibia–Reptilia 20:173–184
Rödel M-O, Spieler M, Grabow K, Böckheler C (1995) Hemisus marmoratus (Peters, 1854) (Anura: Hemisotidae), Fortpflanzungsstrategien eines Savannenfrosches. Bonn Zool Beitr 45:191–207
Ryszkowski L, Truszkowski J (1975) Estimation of the abundance and biomass of transformed amphibians in a field pond. Bull Pol Acad Sci Biol Sci 23:109–113
Schmidt BR, Schaub M, Anholt BR (2002) Why you should use capture-recapture methods when estimating survival and breeding probabilities: on bias, temporary emigration, overdispersion, and common toads. Amphibia–Reptilia (23):375–388
Semlitsch RD (1993) Effects of different predators on the survival and development of tadpoles from the hybridogenetic Rana esculenta complex. Oikos 67:40–46
Semlitsch RD (2002) Critical elements for biologically based recovery plans of aquatic-breeding amphibians. Conserv Biol 16:619–629
Vonesh JR, De la Cruz O (2002) Complex life cycles and density dependence: assessing the contribution of egg mortality to amphibian declines. Oecologia 133:25–333
Wake DB (1991) Declining amphibian populations. Science 253:860
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46 [Suppl]:120–139
Wood KV, Nichols JD, Percival HF, Hines JE (1998) Size–sex variation in survival rates and abundance of pig frogs, Rana grylio, in northern Florida wetlands. J Herpetol 32:527–535
Acknowledgements
We thank the government of the Ivory Coast for granting the necessary research permits to conduct this study. For help with field work we thank Minnattallah Boutros, Alexandra Kaminsky, Martin Kaltenpoth, Koffi Kouadio, Christiane Meyer, Matthias Mösl, Claudia Müller, and Annette Schmidt. We thank Benedikt Schmidt and Michael Schaub for comments on the manuscript. This study was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 251/B3) and a seed grant from the Declining Amphibian Populations Task Force.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grafe, T.U., Kaminsky, S.K., Bitz, J.H. et al. Demographic dynamics of the afro-tropical pig-nosed frog, Hemisus marmoratus: effects of climate and predation on survival and recruitment. Oecologia 141, 40–46 (2004). https://doi.org/10.1007/s00442-004-1639-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1639-7