Abstract
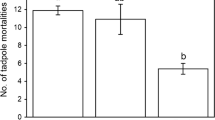
The effect of a predator on the abundance of a prey species depends upon the predator’s abundance and its ability to capture that prey. The objectives of this research were to evaluate the community structure of predators of green treefrog (Hyla cinerea) tadpoles across habitat types and evaluate the effectiveness of individual predators on H. cinerea tadpoles. Correspondence and cluster analyses of predator frequencies across 23 aquatic habitats indicated that the majority of variance in predator communities was due to a division between permanent and temporary habitats. Experimental work demonstrated that survival of the smallest H. cinerea tadpoles was significantly lower than survival of medium and large tadpoles with the most effective predators, indicating that H. cinerea tadpoles attain a refuge from predation at larger body sizes. We combined the effectiveness of predators in experiments with the abundance of each predator species from the predator community survey to demonstrate that predation pressure on H. cinerea tadpoles is higher in temporary ponds. This pattern may explain in part why this species generally breeds successfully only in permanent habitats. It also confirms that discussions about an increasing gradient of predation pressure from temporary to permanent aquatic habitats should be restricted to individual prey species for which such a gradient has been demonstrated.




Similar content being viewed by others
References
Bleckmann H, Lotz T (1987) The vertebrate-catching behaviour of the fishing spider Dolomedestriton (Aranae, Pisauridae). Anim Behav 35:641–651
Blouin MS (1990) Evolution of palatability differences between closely-related treefrogs. J Herpetol 24:309–311
Brodie ED Jr, Formanowicz DR (1987) Antipredator mechanisms of larval anurans: protection of palatable individuals. Herpetologica 43:369–373
Chick JH, Jordan F, Smith JP, McIvor CC (1992) A comparison of four enclosure traps and methods used to sample fishes in aquatic macrophytes. J Freshwater Ecol 7:353–361
Cronin JT, Travis J (1986) Size-limited predation on larval Rana aerolata (Anura: Ranidae) by two species of backswimmers (Insecta: Heteroptera: Notonectidae). Herpetologica 42:171–174
Formanowicz DR, Brodie ED Jr (1982) Relative palatabilities of members of a larval amphibian community. Copeia 1982:91–97
Garton JS, Brandon RA (1975) Reproductive ecology of the green treefrog Hyla cinerea in Southern Illinois (Anura: Hylidae). Herpetologica 31:150–161
Heidinger RC (1975) Life history and biology of the largemouth bass. In: Clepper H (ed) Black bass biology and management. Sport Fishing Institute, Washington, pp 11–20
Jordan F, Coyne S, Trexler JC (1997) Sampling fishes in vegetated habitats: effects of habitat structure and sampling characteristics of the 1-m2 throw trap. Trans Am Fish Soc 126:1012–1020
Kats LB, Petranka JW, Sih A (1988) Antipredator defenses and the persistence of amphibian larvae with fishes. Ecology 69:1865–1870
Laska MS, Wootton JT (1994) Theoretical concepts and empirical approaches to measuring interaction strength. Ecology 79:461–476
Legendre P, Legendre P (1998) Numerical ecology. Developments in environmental modeling, 20. Elsevier, Amsterdam
Leips J, Travis J (1999) The comparative expression of life-history traits and its relationship to the numerical dynamics of four populations of least killifish. J Anim Ecol 68:595–616
Leips J, McManus MG, Travis J (2000) Response of treefrog larvae to drying ponds: comparing temporary and permanent pond breeders. Ecology 81:2997–3008
McPeek MA (1990) Determination of species composition in the Enallagma damselfly assemblages of permanent lakes. Ecology 71:83–98
McPeek MA (1997) Measuring phenotypic selection on an adaptation: lamellae of damselflies experiencing dragonfly predation. Evolution 51:459–466
Morin PJ (1983) Predation, competition, and the composition of larval anuran guilds. Ecol Monogr 53:119–138
Morin PJ (1995) Functional redundancy, non-additive interactions, and supply-side dynamics in experimental pond communities. Ecology 76:133–149
Mount RH (1975) The reptiles and amphibians of Alabama. Auburn, Auburn
Paine RT (1974) Intertidal community structure: experimental studies on the relationship between a dominant competitor and its principal predator. Oecologia 15:93–120
Relyea RA (2002) Local population differences in phenotypic plasticity: predator-induced changes in wood frog tadpoles. Ecol Monogr 72:77–93
Resetarits WJ Jr, Wilbur HM (1991) Calling site choice by Hyla chrysoscelis: effect of predators, competitors, and oviposition sites. Ecology 72:778–786
Schemske DW, Horvitz CC (1984) Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science 225:519–521
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators on prey. Trends Ecol Evol 13:350–355
Travis J, Keen WH, Juilianna J (1985) The role of relative body size in a predator–prey relationship between dragonfly naiads and larval anurans. Oikos 45:59–65
Turner AM, Trexler JC (1997) Sampling aquatic invertebrates from marshes: evaluating the options. J N Am Benthol Soc 16:694–709
Van Buskirk J, McCollum SA (1999) Plasticity and selection explain variation in tadpole phenotype between ponds with different predator composition. Oikos 85:31–39
Wellborn GA, Skelly DK, Werner EE (1996) Mechanisms creating community structure across a freshwater habitat gradient. Annu Rev Ecol Syst 27:337–363
Werner EE, McPeek MA (1994) Direct and indirect effects of predators on two anuran species across an environmental gradient. Ecology 75:1368–1382
Wilbur HM (1997) Experimental ecology of food webs: complex systems in temporary ponds. Ecology 78:2279–2302
Woodward BD (1983) Predator–prey interactions and breeding-pond use of temporary pond species in a desert anuran community. Ecology 64:1549–1555
Wright AH (1932) Life-histories of the frogs of Okefinokee Swamp, Georgia. Comstock, Cornell University Press, Ithaca
Acknowledgements
We thank M. Aresco, R. Fuller, B. Hale, B. Storz, and members of the 2001 FSU Advanced Field Biology class for assistance during the field censuses. J. Gunzburger, E. Gunzburger, and M. Aresco assisted with experiments at the greenhouse. We thank E. Walters, P. Richards, T. Miller, and F. James for comments on an earlier draft of this manuscript. Experiments and field sampling were conducted under FSU ACUC Protocol # 0115. We acknowledge the support of the National Science Foundation through grant DEB 99–03925 to J.T.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunzburger, M.S., Travis, J. Evaluating predation pressure on green treefrog larvae across a habitat gradient. Oecologia 140, 422–429 (2004). https://doi.org/10.1007/s00442-004-1610-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1610-7