Abstract
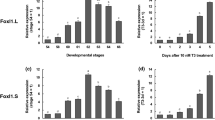
During amphibian intestinal remodeling, thyroid hormone (TH) induces some larval epithelial cells to dedifferentiate into adult stem cells, which newly generate the absorptive epithelium analogous to the mammalian epithelium. To clarify molecular mechanisms underlying adult epithelial development, we here focus on TH response genes that are associated with the canonical Wnt pathway. Our quantitative reverse transcription plus polymerase chain reaction and immunohistochemical analyses indicate that all of the genes examined, including β-catenin, c-Myc and secreted frizzle-related protein 2 (SFRP2), are up-regulated in Xenopus laevis intestine during both natural and TH-induced metamorphosis. Moreover, immunoreactivity for nuclear β-catenin becomes detectable in adult stem cells from the start of their appearance and then increases in intensity in adult epithelial primordia derived from the stem cells, which actively proliferate and coexpress Wnt target genes c-Myc and LGR5. These expression profiles strongly suggest the involvement of the canonical Wnt pathway in the maintenance and/or proliferation of adult stem/progenitor cells. More importantly, by using organ cultures of the tadpole intestine, we have experimentally shown that the addition of exogenous SFRP2 protein to the culture medium promotes cell proliferation of the adult epithelial primordia, whereas inhibition of endogenous SFRP2 by its antibody suppresses their proliferation. The inhibition of SFRP2 suppresses larval epithelial changes in shape from simple columnar to stem-cell-like roundish cells, resulting in the failure of epithelial dedifferentiation. Thus, TH-up-regulated SFRP2 in the postembryonic intestine promotes adult stem cell development, possibly by acting as an agonist of both canonical and non-canonical Wnt signaling.






Similar content being viewed by others
References
Barker N, Tan S, Clevers H (2013) Lgr proteins in epithelial stem cell biology. Development 140:2484–2494
Behrens J, Lustig B (2004) The Wnt connection to tumorigenesis. Int J Dev Biol 48:477–487
Bjerknes M, Cheng H (1981) The stem-cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat 160:77–91
Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J (2008) Beyond Wnt inhibition: new functions of secreted frizzled-related proteins in development and disease. J Cell Sci 121:737–746
Brown AR, Simmen RC, Simmen FA (2013) The role of thyroid hormone signaling in the prevention of digestive system cancers. Int J Mol Sci 14:16240–16257
Buchholz DR, Heimeier RA, Das B, Washington T, Shi Y-B (2007) Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol 303:576–590
Cheng H, Bjerknes M (1985) Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat Rec 211:420–426
Chien AJ, Conrad WH, Moon RT (2009) A Wnt survival guide: from flies to human disease. J Invest Dermatol 129:1614–1627
Clevers H, Nusse R (2012) Wnt/β-catenin signaling and disease. Cell 149:1192–1205
Clevers H, Loh KM, Nusse R (2014) Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346:1248012
Courtwright A, Siamakpour-Reihani S, Arbiser JL, Banet N, Hilliard E, Fried L, Livasy C, Ketelsen D, Nepal DB, Perou CM, Patterson C, Klauber-Demore N (2009) Secreted frizzle-related protein 2 stimulates angiogenesis via a calcineurin/ NFAT signaling pathway. Cancer Res 69:4621–4628
Crosnier C, Stamataki D, Lewis J (2006) Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 7:349–359
Dodd MHI, Dodd JM (1976) The biology of metamorphosis. In: Lofts B (ed) Physiology of the amphibia. Academic Press, New York, pp 467–599
Fevr T, Robine S, Louvard D, Huelsken J (2007) Wnt/β-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol 27:7551–7559
Flanagan DJ, Phesse TJ, Barker N, Schwab RH, Amin N, Malaterre J, Stange DE, Nowell CJ, Currie SA, Saw JT, Beuchert E, Ramsay RG, Sansom OJ, Ernst M, Clevers H, Vincan E (2015) Frizzled7 functions as a Wnt receptor in intestinal epithelial Lgr5(+) stem cells. Stem Cell Rep 4:759–767
Fujimoto K, Matsuura K, Hu-Wang E, Lu R, Shi Y-B (2012) Thyroid hormone activates protein arginine methyltransferase 1 expression by directly inducing c-Myc transcription during Xenopus intestinal stem cell development. J Biol Chem 287:10039–10050
Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ (2011) The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci U S A 108:10585–10590
Hasebe T, Kajita M, Shi Y-B, Ishizuya-Oka A (2008) Thyroid hormone-up-regulated hedgehog interacting protein is involved in larval-to-adult intestinal remodeling by regulating sonic hedgehog signaling pathway in Xenopus laevis. Dev Dyn 237:3006–3015
Heimeier RA, Das B, Buchholz DR, Fiorentino M, Shi Y-B (2010) Studies on Xenopus laevis intestine reveal biological pathways underlying vertebrate gut adaptation from embryo to adult. Genome Biol 11:R55
Hourdry J, Dauca M (1977) Cytological and cytochemical changes in the intestinal epithelium during amphibian metamorphosis. Int Rev Cytol (Suppl) 5:337–385
Ishizuya-Oka A, Hasebe T (2013) Establishment of intestinal stem cell niche during amphibian metamorphosis. Curr Top Dev Biol 103:305–327
Ishizuya-Oka A, Shimozawa A (1991) Induction of metamorphosis by thyroid hormone in anuran small intestine cultured organotypically in vitro. In Vitro Cell Dev Biol 27A:853–857
Ishizuya-Oka A, Ueda S (1996) Apoptosis and cell proliferation in the Xenopus small intestine during metamorphosis. Cell Tissue Res 286:467–476
Ishizuya-Oka A, Shimizu K, Sakakibara S, Okano H, Ueda S (2003) Thyroid hormone-upregulated expression of Musashi-1 is specific for progenitor cells of the adult epithelium during amphibian gastrointestinal remodeling. J Cell Sci 116:3157–3164
Ishizuya-Oka A, Hasebe T, Buchholz DR, Kajita M, Fu L, Shi Y-B (2009) Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. FASEB J 23:2568–2575
Ishizuya-Oka A, Kajita M, Hasebe T (2014) Thyroid hormone-regulated Wnt5a/Ror2 signaling is essential for dedifferentiation of larval epithelial cells into adult stem cells in the Xenopus laevis intestine. PLoS ONE 9:e107611
Katoh M, Katoh M (2007) Wnt signaling pathway and stem cell signaling network. Clin Cancer Res 13:4042–4025
Kawano Y, Kypta R (2003) Secreted antagonists of the Wnt signalling pathway. J Cell Sci 116:2627–2634
Kikuyama S, Kawamura K, Tanaka S, Yamamoto K (1993) Aspects of amphibian metamorphosis: hormonal control. Int Rev Cytol 145:105–148
Kim B, Mao MJ, Taketo MM, Shivdasani RA (2007) Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology 133:529–538
Korinek V, Barker N, Moerer P, Donselaar E van, Huls G, Peters PJ, Clevers H (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19:379–383
Kress E, Rezza A, Nadiar J, Samarut J, Plateroti M (2009) The frizzled-related sfrp2 gene is a target of thyroid hormone receptor α1 and activates β-catenin signaling in mouse intestine. J Biol Chem 284:1234–1241
Li Y, Lu W, King TD, Liu CC, Bijur GN, Bu G (2010) Dkk1 stabilizes Wnt co-receptor Lrp6: implication for Wnt ligand-induced Lrp6 down-regulation. PLoS ONE 5:e11014
Matsuyama M, Aizawa S, Shimono A (2009) Sfrp controls apicobasal polarity and oriented cell division in developing gut epithelium. PLoS Genet 5:e1000427
McAvoy JW, Dixon KE (1977) Cell proliferation and renewal in the small intestinal epithelium of metamorphosing and adult Xenopus laevis. J Exp Zool 202:129–138
Muncan V, Heijmans J, Krasinski SD, Buller NV, Wildenberg ME, Meisner S, Radonjic M, Stapleton KA, Lamers WH, Biemond I, Bergh Weerman MA van den, O'Carroll D, Hardwick JC, Hommes DW, Brink GR van den (2011) Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun 2:452
Nakamura T, Nakamura T, Matsumoto K (2008) The functions and possible significance of Kremen as the gatekeeper of Wnt signalling in development and pathology. J Cell Mol Med 12:391–408
Nieuwkoop PD, Faber J (1967) Normal table of Xenopus laevis (Daudin). North-Holland, Amsterdam
Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y (2003) The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8:645–654
Sato T, Clevers H (2013) Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340:1190–1194
Sato T, Vries RG, Snippert HJ, Wetering M van de, Barker N, Stange DE, Es JH van, Abo A, Kujala P, Peters PJ, Clevers H (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265
Sato T, Es JH van, Snippert HJ, Stange DE, Vries RG, Born M van den, Barker N, Shroyer NF, Wetering M van de, Clevers H (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469:415–418
Satoh W, Matsuyama M, Takemura H, Aizawa S, Shimono A (2008) Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/β-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis 46:92–103
Schneider S, Steinbeisser H, Warga RM, Hausen P (1996) β-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev 57:191–198
Shi Y-B (1999) Amphibian metamorphosis: from morphology to molecular biology. Wiley, New York
Shi Y-B, Ishizuya-Oka A (1996) Biphasic intestinal development in amphibians: embryogenesis and remodeling during metamorphosis. Curr Top Dev Biol 32:205–235
Shi Y-B, Liang VC (1994) Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. Biochim Biophys Acta 1217:227–228
Sirakov M, Plateroti M (2011) The thyroid hormones and their nuclear receptors in the gut: from developmental biology to cancer. Biochim Biophys Acta 1812:938–946
Sirakov M, Skah S, Nadjar J, Plateroti M (2013) Thyroid hormone’s action on progenitor/stem cell biology: new challenge for a classic hormone? Biochim Biophys Acta 1830:3917–3927
Skah S, Nadjar J, Sirakov M, Plateroti M (2015) The secreted frizzled-related protein 2 modulates cell fate and the Wnt pathway in the murine intestinal epithelium. Exp Cell Res 330:56–65
Snippert HJ, Flier LG van der, Sato T, van Es JH, Born M, Kroon-Veenboer C van den, Barker N, Klein AM, Rheenen J van, Simons BD, Clevers H (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143:134–144
Sun G, Hasebe T, Fujimoto K, Lu R, Fu L, Matsuda H, Kajita M, Ishizuya-Oka A, Shi Y-B (2010) Spatio-temporal expression profile of stem cell-associated gene LGR5 in the intestine during thyroid hormone-dependent metamorphosis in Xenopus laevis. PLoS ONE 5:e13605
Sun G, Heimeier RA, Fu L, Hasebe T, Das B, Ishizuya-Oka A, Shi Y-B (2013) Expression profiling of intestinal tissues implicates tissue-specific genes and pathways essential for thyroid hormone-induced adult stem cell development. Endocrinology 154:4396–4407
Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Engeland M van, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG, Baylin SB (2004) Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet 36:417–422
Wang K, Zhang Y, Li X, Chen L, Wang H, Wu J, Zheng J, Wu D (2008) Characterization of the Kremen-binding site on Dkk1 and elucidation of the role of Kremen in Dkk-mediated Wnt antagonism. J Biol Chem 283:23371–23375
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by the Grants-in-Aid for Scientific Research (C) from the Ministry of Education, Science and Culture of Japan (Grant No. 25440160 to T. H. and No. 24570078 to A. I.-O.).
Rights and permissions
About this article
Cite this article
Hasebe, T., Fujimoto, K., Kajita, M. et al. Thyroid hormone activates Wnt/β-catenin signaling involved in adult epithelial development during intestinal remodeling in Xenopus laevis . Cell Tissue Res 365, 309–318 (2016). https://doi.org/10.1007/s00441-016-2396-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2396-8