Abstract
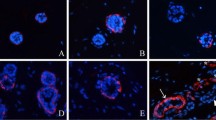
We have examined the changes of keratins and alpha-SMA at various time points in order to investigate the development and differentiation of eccrine sweat gland cells during the course of three-dimensional (3D) reconstitution. Mixtures of eccrine sweat gland cells and Matrigel were injected subcutaneously into the inguinal regions of nude mice. At 1, 2, 4, 6, 8, 14, 21, 28, 35, and 42 days post-implantation, Matrigel plugs were removed and immunostained. We found that during 3D reconstitution, keratin and alpha-SMA expression changed in a time-dependent manner. At day 1, all cells stained positively for keratin isoforms K5, K14, and K15, with the staining intensity of K15 being weak and K5 and K14 being strong, but none of the cells displayed K7, K8, or alpha-SMA. As time progressed, spheroid-like structures formed with the inner layer acquiring K7 and K8, but losing K5 and K14 expression, and the outer layer acquiring alpha-SMA expression, but losing K15 expression. K8 expression was first noted at day 14, and K7 and alpha-SMA at day 21. The loss of K15 expression was first noted at day 14, K14 at day 21, and K5 at day 28. At 28, 35, and 42 days, the spheroid-like structures could be distinguished, by immunohistochemistry, as having secretory coil-like and coiled duct-like structures. We conclude that the changes in expression of keratins and alpha-SMA in 3D-reconstituted eccrine sweat glands are similar to those of native eccrine sweat glands, indicating that the 3D reconstitution of sweat glands provides an excellent model for studying the development, cytodifferentiation, and regulation of eccrine sweat glands.







Similar content being viewed by others
References
Ansai SI, Katagata Y, Yoshikawa KI, Hozumi Y, Aso K (1993) Keratin specificity analyses of eight anti-keratin monoclonal antibodies, and their immunostaining patterns in normal skin using formalin-fixed and paraffin-embedded tissue specimens. Arch Dermatol Res 285:6–12
Fuchs E, Green H (1980) Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 19:1033–1042
Grice EA, Segre JA (2011) The skin microbiome. Nat Rev Microbiol 9:244–253
Jackson BW, Grund C, Schmid E, Burki K, Franke WW, Illmensee K (1980) Formation of cytoskeletal elements during mouse embryogenesis. Intermediate filaments of the cytokeratin type and desmosomes in preimplantation embryos. Differentiation 17:161–179
Kawasaki Y, Imaizumi T, Matsuura H, Ohara S, Takano K, Suyama K, Hashimoto K, Nozawa R, Suzuki H, Hosoya M (2008) Renal expression of alpha-smooth muscle actin and c-Met in children with Henoch-Schonlein purpura nephritis. Pediatr Nephrol 23:913–919
Komine M, Hattori N, Tamaki K (2000) Eccrine syringofibroadenoma (Mascaro): an immunohistochemical study. Am J Dermatopathol 22:171–175
Langbein L, Reichelt J, Eckhart L, Praetzel-Wunder S, Kittstein W, Gassler N, Schweizer J (2013) New facets of keratin K77: interspecies variations of expression and different intracellular location in embryonic and adult skin of humans and mice. Cell Tissue Res 354:793–812
Li H, Chen L, Zeng S, Li X, Zhang X, Lin C, Zhang M, Xie S, He Y, Shu S, Yang L, Tang S, Fu X (2015a) Matrigel basement membrane matrix induces eccrine sweat gland cells to reconstitute sweat gland-like structures in nude mice. Exp Cell Res 332:67–77
Li H, Zhang X, Zeng S, Li X, Zhang B, Chen L, Lin C, Zhang M, Tang S, Fu X (2015b) Combination of keratins and alpha-smooth muscle actin distinguishes secretory coils from ducts of eccrine sweat glands. Acta Histochem 117:275–278
Lu C, Fuchs E (2014) Sweat gland progenitors in development, homeostasis, and wound repair. Cold Spring Harb Perspect Med 4:a015222
Lu CP, Polak L, Rocha AS, Pasolli HA, Chen SC, Sharma N, Blanpain C, Fuchs E (2012) Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 150:136–150
Moll I, Moll R (1991) Comparative cytokeratin analysis of sweat gland ducts and eccrine poromas. Arch Dermatol Res 283:300–309
Moll I, Moll R (1992) Changes of expression of intermediate filament proteins during ontogenesis of eccrine sweat glands. J Invest Dermatol 98:777–785
Moll R, Franke WW, Volc-Platzer B, Krepler R (1982a) Different keratin polypeptides in epidermis and other epithelia of human skin: a specific cytokeratin of molecular weight 46,000 in epithelia of the pilosebaceous tract and basal cell epitheliomas. J Cell Biol 95:285–295
Moll R, Moll I, Wiest W (1982b) Changes in the pattern of cytokeratin polypeptides in epidermis and hair follicles during skin development in human fetuses. Differentiation 23:170–178
Moll R, Divo M, Langbein L (2008) The human keratins: biology and pathology. Histochem Cell Biol 129:705–733
Montagna W, Chase HB, Lobitz WC Jr (1953) Histology and cytochemistry of human skin. IV. The eccrine sweat glands. J Invest Dermatol 20:415–423
Nagamoto T, Eguchi G, Beebe DC (2000) Alpha-smooth muscle actin expression in cultured lens epithelial cells. Invest Ophthalmol Vis Sci 41:1122–1129
Saga K (2002) Structure and function of human sweat glands studied with histochemistry and cytochemistry. Prog Histochem Cytochem 37:323–386
Schon M, Benwood J, O’Connell-Willstaedt T, Rheinwald JG (1999) Human sweat gland myoepithelial cells express a unique set of cytokeratins and reveal the potential for alternative epithelial and mesenchymal differentiation states in culture. J Cell Sci 112:1925–1936
Shibasaki M, Wilson TE, Crandall CG (2006) Neural control and mechanisms of eccrine sweating during heat stress and exercise. J Appl Physiol 100:1692–1701
Taylor DK, Bubier JA, Silva KA, Sundberg JP (2012) Development, structure, and keratin expression in C57BL/6J mouse eccrine glands. Vet Pathol 49:146–154
Van Muijen GN, Warnaar SO, Ponec M (1987) Differentiation-related changes of cytokeratin expression in cultured keratinocytes and in fetal, newborn, and adult epidermis. Exp Cell Res 171:331–345
Wong DJ, Chang HY (2009) Skin tissue engineering. In: Bhatia S, Polak J, The Stem Cell Research Community (eds) StemBook, Harvard Stem Cell Institute, Cambridge, Mass. doi:10.3824/stembook.1.44.1
Zhang CP, Fu XB (2008) Therapeutic potential of stem cells in skin repair and regeneration. Chin J Traumatol 11:209–221
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest’s statement
We declare that we have no competing financial, personal, or other relationships with any individuals or other organizations.
Additional information
The work described in this manuscript was supported in part by the National Natural Science Foundation of China (81471882, 81071551) and the Natural Science Foundation of Guangdong Province, China (2014A030313476).
Rights and permissions
About this article
Cite this article
Li, H., Li, X., Zhang, B. et al. Changes in keratins and alpha-smooth muscle actin during three-dimensional reconstitution of eccrine sweat glands. Cell Tissue Res 365, 113–122 (2016). https://doi.org/10.1007/s00441-016-2357-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2357-2