Abstract
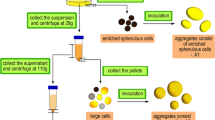
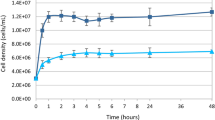
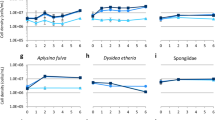
Marine sponges (Porifera) are the best source of marine bioactive metabolites for drug discovery and development, although the sustainable production of most sponge-derived metabolites remains a difficult task. In vitro cultivation of sponge cells in bioreactors has been proposed as a promising technology. However, no continuous cell line has as yet been developed. Archaeocytes are considered to be toti/multipotent stem cells in sponges and, when purified, may allow the development of continuous sponge cell lines. As a prerequisite, we have developed a novel four-step protocol for the purification of archaeocytes from a marine sponge, Hymeniacidon perleve: (1) differential centrifugation to separate large sponge cells including archaeocytes; (2) selective agglomeration in low-Ca2+/Mg2+ artificial seawater in which living archaeocytes form small loose aggregates with some pinacocytes and collencytes; (3) differential adherence to remove anchorage-dependent pinacocytes, collencytes and other mesohyl cells; (4) Ficoll-Vrografin density gradient centrifugation to purify archaeocytes. The final purity of archaeocytes is greater than 80%. The proliferation potential of the archaeocytes has been demonstrated by high levels of BrdU incorporation, PCNA expression and telomerase activity. In 4-day primary cultures, the purified archaeocytes show a 2.5-fold increase in total cell number. This study opens an important avenue towards developing sponge cell cultures for the commercial exploitation of sponge-derived drugs.












Similar content being viewed by others
References
Almendral JM, Huebsch D, Blundell PA, Macdonald-Bravo H, Bravo R (1987) Cloning and sequence of the human nuclear protein cyclin: homology with DNA-binding proteins. Proc Natl Acad Sci USA 84:1575–1579
Bauer GA, Burgers PMJ (1988) The yeast analog of mammalian cyclin/proliferating-cell nuclear antigen interacts with mammalian DNA polymerase. Proc Natl Acad Sci USA 85:7506–7510
Bayne CJ (1998) Invertebrate cell culture considerations: insects, ticks, shellfish, and worms. In: Mather JP, Barnes D (eds) Methods in cell biology, vol 57. Academic Press, New York, pp 187–201
Bergquist PR (1978) Sponges. University of California Press, Berkeley
Berry L (2003) Soaking up the limelight. Nature 421:791
Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR (2005) Marine natural products. Nat Prod Rep 22:15–61
Boury-Esnault N (1977) A cell type in sponge involved in the metabolism of glycogen: the gray cells. Cell Tissue Res 175:523–539
Bravo R (1986) Synthesis of the nuclear protein cyclin (PCNA) and its relationship with DNA replication. Exp Cell Res 163:287–293
Celis JE, Madsen P, Celis A, Nielsen HV, Gesser B (1987) Cyclin (PCNA, auxiliary protein of DNA polymerase δ) is a central component of the pathway(s) leading to DNA replication and cell division. FEBS Lett 220:1–7
Corredor JE, Wilkinson CR, Vicente VP, Morell JM, Otero E (1988) Nitrate release by Caribbean reef sponges. Limnol Oceanogr 33:114–120
Custódio MR, Prokic I, Steffen R, Koziol C, Borojevic R, Brummer F, Nickel M, Müller WEG (1998) Primmorphs generated from dissociated cells of the sponge Suberites domuncula: a model system for studies of cell proliferation and cell death. Mech Ageing Dev 105:45–59
Custódio MR, Hajdu E, Muricy G (2004) Cellular dynamics of in vitro allogeneic reactions of Hymeniacidon heliophila (Demospongiae: Halichondrida). Mar Biol 144:999–1010
Daidoji H, Takasaki Y, Nakane PK (1992) Proliferating cell nuclear antigen (PCNA/cyclin) in plant proliferating cells: immunohistochemical and quantitative analysis using autoantibody and murine monoclonal antibodies to PCNA. Cell Biochem Funct 10:123–132
De Rosa S, De Caro S, Iodice C, Tommonaro G, Stefanov K, Popov S (2003) Development in primary cell culture of Demosponges. J Biotechnol 100:119–125
De Sutter D, Van de Vyver G (1977) Aggregative properties of different cell types of the fresh-water sponge Ephydatia fluviatilis isolated on Ficoll gradients. Rouxs Arch 181:151–161
Faulkner DJ (1998) Marine natural products. Nat Prod Rep 15:113–158
Flowers AE, Garson MJ, Webb RI, Dumdei EJ, Charan RD (1998) Cellular origin of chlorinated diketopiperazines in the dictyoceratid sponge Dysidea herbacea (Keller). Cell Tissue Res 292:597–607
Fusetani N (2000) Drugs from the sea. Karger, Basel
Garson MJ, Flowers AE, Webb RI, Charan RD, McCaffrey EJ (1998) A sponge/dinoflagellate association in the haplosclerid sponge Haliclona sp.: cellular origin of cytotoxic alkaloids by Percoll density gradient fractionation. Cell Tissue Res 293:365–373
Gratzner HG (1982) Monoclonal antibody to 5-bromo and 5-iododeoxyuridine: a new reagent for detection of DNA replication. Science 218:474–475
Ilan M, Contini H, Carmeli S, Rinkevich B (1996) Progress towards cell cultures from a marine sponge that produces bioactive compounds. J Mar Biotechnol 4:145–149
Imsiecke G, Steffen R, Custodio MR, Borojevic R, Müller WEG (1995) Formation of spicules by sclerocytes from the freshwater sponge Ephydatia muelleri in short-term cultures in vitro. In Vitro Cell Dev Biol 31:528–535
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL (1994) Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011–2015
Koziol C, Borojevic R, Steffen R, Müller WEG (1998) Sponges (Porifera) model systems to study the shift from immortal to senescent somatic cells: the telomerase activity in somatic cells. Mech Ageing Dev 100:107–120
Le Pennec G, Perovic S, Ammar MSA, Grebenjuk VA, Steffen R, Brümmer F, Müller WEG (2003) Cultivation of primmorphs from the marine sponge Suberites domuncula: morphogenetic potential of silicon and iron. J Biotechnol 100:93–108
Matsumoto K, Moriuchi T, Koji T, Nakane PK (1987) Molecular cloning of cDNA coding for rat proliferating cell nuclear antigen (PCNA)/cyclin. EMBO J 6:637–642
Moriuchi T, Matsumoto K, Koji T, Nakane PK (1986) Molecular cloning and nucleotide sequence analysis of rat PCNA/cyclin cDNA. Nucleic Acids Symp Ser 17:117–120
Müller WEG, Schäcke H (1996) Characterization of the receptor protein-tyrosine kinase genes from the marine sponge Geodia cydonium. Prog Mol Subcell Biol 17:183–208
Müller WEG, Wiens M, Batel R, Steffen R, Schröder HC, Borojevic R, Custódio MR (1999) Establishment of a primary cell culture from a sponge: primmorphs from Suberites domuncula. Mar Ecol Prog Ser 178:205–219
Müller WEG, Böhm M, Batel R, De Rosa S, Tommonaro G, Müller IM, Schröder HC (2000) Application of cell culture for the production of bioactive compounds from sponges: synthesis of avarol by primmorphs from Dysidea avara. J Nat Prod 63:1077–1081
Naganuma T, Degan BM, Horikoshi K, Morse DE (1994) Myogenesis in primary cell cultures from larvae of the abalone, Haliotis rufescens. Mol Mar Biol Biotech 3:131–140
Osinga R, Tramper J, Wijffels RH (1999) Cultivation of marine sponges. Mar Biotechnol 1:509–532
Paunesku T, Mittal S, Protic M, Oryhon J, Korolev SV, Joachimiak A, Woloschak GE (2001) Proliferating cell nuclear antigen (PCNA): ringmaster of the genome. Int J Radiat Biol 77:1007–1021
Piatyszek MA, Kim NW, Weinrich SL, Hiyama K, Hiyama E, Wright WE, Shay JW (1995) Detection of telomerase activity in human cells and tumors by a telomeric repeat amplification protocol (TRAP). Methods Cell Sci 17:1–15
Pomponi SA, Willoughby R (1994) Sponge cell culture for production of bioactive metabolites. In: van Soest SR, Balkema AA (eds) Sponges in time and space. Rotterdam, Brookfield, pp 395–400
Prelich G, Tan CK, Kostura M, Mathews MB, So AG, Downey KM, Stillman B (1987) Functional identify of proliferating cell nuclear antigen and a DNA polymerase-δ auxiliary protein. Nature 326:517–520
Rinkevich B (1999) Cell cultures from marine invertebrates: obstacles, new approaches and recent improvements. J Biotechnol 70:133–153
Rinkevich B (2005) Marine invertebrate cell cultures: new millennium trends. Mar Biotechnol 7:429–439
Rinkevich B, Rabinowitz C (1994) Acquiring embryo-derived cell cultures and aseptic metamorphosis of larvae from the colonial protochordate Botryllus schlosseri. Invertebr Reprod Dev 25:59–72
Rosenfield A, Kern FG, Keller BJ (1994) Invertebrate neoplasia: initiation and promotion mechanisms. NOAA Technical Memorandum NMFS-NE-107:31
Salomon CE, Deerinck T, Ellisman MH, Faulkner DJ (2001) The cellular localization of dercitamide in the Palauan sponge Oceanapia sagittaria. Mar Biol 139:313–319
Simpson TL (1984) The cell biology of sponges. Springer, Berlin Heidelberg New York
Suzuka I, Daidoji H, Matsuoka M, Kadowaki K, Takasaki Y, Nakane PK, Moriuchi T (1989) Gene for proliferating-cell nuclear antigen (DNA polymerase delta auxiliary protein) is present in both mammalian and higher plant genomes. Proc Natl Acad Sci USA 86:3189–3193
Uriz MJ, Turon X, Galera J, Tur JM (1996) New light on the cell location of avarol within the sponge Dysidea avara (Dendroceratida). Cell Tissue Res 285:519–527
Willoughby R, Pomponi SA (2000) Quantitative assessment of marine sponge cells in vitro: development of improved growth medium. In Vitro Cell Dev Biol Animal 36:194–200
Wilkinson CR (1987) Interocean differences in size and nutrition of coral reef sponge populations. Science 236:1654–1657
Yin CQ, Humphreys T (1996) Acute cytotoxic allogeneic histoincompatibility reactions involving gray cells in the marine sponge, Callyspongeia diffusa. Biol Bull 191:159–167
Zhang W, Zhang XY, Cao XP, Xu JY, Zhao QY, Yu XJ, Jin MF, Deng MC (2003a) Optimizing the formation of in vitro sponge primmorphs from the Chinese sponge Stylotella agminata (Ridley). J Biotechnol 100:161–168
Zhang XY, Cao XP, Zhang W, Yu XJ, Jin MF (2003b) Primmorphs from archaeocytes—dominant cell population of the sponge Hymeniacidon perleve: improved cell proliferation and spiculogenesis. Biotechnol Bioeng 84:583–590
Zhao QY, Jin MF, Müller WEG, Zhang W, Yu XJ, Deng MC (2003) Attachment of marine sponge cells of Hymeniacidon perleve on microcarriers. Biotechnol Prog 19:1569–1573
Zhao QY, Zhang W, Jin MF, Yu XJ, Deng MC (2005) Formulation of a basal medium for primary cell culture of the marine sponge Hymeniacidon perleve. Biotechnol Prog 21:1008–1012
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors are grateful for the financial support of the Chinese Academy of Sciences under the “100 Talent Project”, the “Innovation Fund” from the Dalian Institute of Chemical Physics, the “Hi-Tech Research and Development Program of China” (2001AA620404), and the European Commission (project: Silicon Biotechnology).
Rights and permissions
About this article
Cite this article
Sun, L., Song, Y., Qu, Y. et al. Purification and in vitro cultivation of archaeocytes (stem cells) of the marine sponge Hymeniacidon perleve (Demospongiae). Cell Tissue Res 328, 223–237 (2007). https://doi.org/10.1007/s00441-006-0342-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0342-x