Abstract
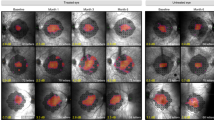
MERTK is an essential component of the signaling network that controls phagocytosis in retinal pigment epithelium (RPE), the loss of which results in photoreceptor degeneration. Previous proof-of-concept studies have demonstrated the efficacy of gene therapy using human MERTK (hMERTK) packaged into adeno-associated virus (AAV2) in treating RCS rats and mice with MERTK deficiency. The purpose of this study was to assess the safety of gene transfer via subretinal administration of rAAV2-VMD2-hMERTK in subjects with MERTK-associated retinitis pigmentosa (RP). After a preclinical phase confirming the safety of the study vector in monkeys, six patients (aged 14 to 54, mean 33.3 years) with MERTK-related RP and baseline visual acuity (VA) ranging from 20/50 to <20/6400 were entered in a phase I open-label, dose-escalation trial. One eye of each patient (the worse-seeing eye in five subjects) received a submacular injection of the viral vector, first at a dose of 150 µl (5.96 × 1010vg; 2 patients) and then 450 µl (17.88 × 1010vg; 4 patients). Patients were followed daily for 10 days at 30, 60, 90, 180, 270, 365, 540, and 730 days post-injection. Collected data included (1) full ophthalmologic examination including best-corrected VA, intraocular pressure, color fundus photographs, macular spectral domain optical coherence tomography and full-field stimulus threshold test (FST) in both the study and fellow eyes; (2) systemic safety data including CBC, liver and kidney function tests, coagulation profiles, urine analysis, AAV antibody titers, peripheral blood PCR and ASR measurement; and (3) listing of ophthalmological or systemic adverse effects. All patients completed the 2-year follow-up. Subretinal injection of rAAV2-VMD2-hMERTK was associated with acceptable ocular and systemic safety profiles based on 2-year follow-up. None of the patients developed complications that could be attributed to the gene vector with certainty. Postoperatively, one patient developed filamentary keratitis, and two patients developed progressive cataract. Of these two patients, one also developed transient subfoveal fluid after the injection as well as monocular oscillopsia. Two patients developed a rise in AAV antibodies, but neither patient was positive for rAAV vector genomes via PCR. Three patients also displayed measurable improved visual acuity in the treated eye following surgery, although the improvement was lost by 2 years in two of these patients. Gene therapy for MERTK-related RP using careful subretinal injection of rAAV2-VMD2-hMERTK is not associated with major side effects and may result in clinical improvement in a subset of patients.





Similar content being viewed by others
References
Diabetic Retinopathy Study Research Group (1985) Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Early treatment diabetic retinopathy study research group. Arch Ophthalmol 103:1796–1806
Abu-Safieh L, Alrashed M, Anazi S, Alkuraya H, Khan AO, Al-Owain M, Al-Zahrani J, Al-Abdi L, Hashem M, Al-Tarimi S (2013) Autozygome-guided exome sequencing in retinal dystrophy patients reveals pathogenetic mutations and novel candidate disease genes. Genome Res 23:236–247
Aleman TS, Jacobson SG, Chico JD, Scott ML, Cheung AY, Windsor EA, Furushima M, Redmond TM, Bennett J, Palczewski K, Cideciyan AV (2004) Impairment of the transient pupillary light reflex in Rpe65(−/−) mice and humans with Leber congenital amaurosis. Invest Ophthalmol Vis Sci 45:1259–1271
Ashtari M, Cyckowski LL, Monroe JF, Marshall KA, Chung DC, Auricchio A, Simonelli F, Leroy BP, Maguire AM, Shindler KS (2011) The human visual cortex responds to gene therapy-mediated recovery of retinal function. J Clin Investig 121:2160
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N (2008) Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med 358:2231–2239
Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ (2015) Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med 372:1887–1897
Berger W, Kloeckener-Gruissem B, Neidhardt J (2010) The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res 29:335–375
Berson EL, Rosner B, Sandberg MA, Hayes K, Nicholson BW, Weigel-DiFranco C, Willett W (1993) A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol 111:761–772
Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, Brockhurst RJ, Hayes K, Johnson EJ, Anderson EJ, Johnson CA, Gaudio AR (2010) Clinical trial of lutein in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol 128:403
Birch DG, Weleber RG, Duncan JL, Jaffe GJ, Tao W (2013) Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am J Ophthalmol 156(283–292):e1
Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY (1993) The lens opacities classification system III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 111:831–836
Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Roman AJ (2009) Vision 1 year after gene therapy for Leber’s congenital amaurosis. N Engl J Med 361:725–727
Cideciyan AV, Jacobson SG, Beltran WA, Sumaroka A, Swider M, Iwabe S, Roman AJ, Olivares MB, Schwartz SB, Komáromy AM (2013) Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci 110:E517–E525
Conlon TJ, Deng W-T, Erger K, Cossette T, J-j Pang, Ryals R, Clément N, Cleaver B, McDoom I, Boye SE (2013) Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum Gene Ther Clin Dev 24:23–28
D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D (2000) Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet 9:645–651
Daiger S, Sullivan L, Bowne S (2013) Genes and mutations causing retinitis pigmentosa. Clin Genet 84:132–141
Dikopf MS, Chow CC, Mieler WF, Tu EY (2013) Cataract extraction outcomes and the prevalence of zonular insufficiency in retinitis pigmentosa. Am J Ophthalmol 156(82–88):e2
Dorn JD, Ahuja AK, Caspi A, da Cruz L, Dagnelie G, Sahel J-A, Greenberg RJ, McMahon MJ, Group AIS (2013) The detection of motion by blind subjects with the epiretinal 60-electrode (Argus II) retinal prosthesis. JAMA Ophthalmol 131:183–189
Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D (2002) Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem 277:17016–17022
Fishman G, Anderson R, Lourenco P (1985) Prevalence of posterior subcapsular lens opacities in patients with retinitis pigmentosa. Br J Ophthalmol 69:263–266
Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J (2013) Gene therapy clinical trials worldwide to 2012—an update. J Gene Med 15:65–77
Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368:1795–1809
Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ (2008) Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 19:979–990
Jacobson SG, Cideciyan AV, Ratnakaram R, Heon E, Schwartz SB, Roman AJ, Peden MC, Aleman TS, Boye SL, Sumaroka A (2012) Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol 130:9–24
Klein M, Birch D (2009) Psychophysical assessment of low visual function in patients with retinal degenerative diseases (RDDs) with the Diagnosys full-field stimulus threshold (D-FST). Doc Ophthalmol 119:217–224
LaVail MM, Yasumura D, Matthes MT, Yang H, Hauswirth WW, Deng WT, Vollrath D (2016) Gene therapy for MERTK-associated retinal degenerations. Adv Exp Med Biol 854:487–493. doi:10.1007/978-3-319-17121-0_65
MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC (2014) Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet 383:1129–1137
Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM (2008) Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 358:2240–2248
Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, G-s Ying, Rossi S (2009) Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet 374:1597–1605
Miller JW (2008) Preliminary results of gene therapy for retinal degeneration. N Engl J Med 358:2282
Patel N, Aldahmesh MA, Alkuraya H, Anazi S, Alsharif H, Khan AO, Sunker A, Al-mohsen S, Abboud EB, Nowilaty SR (2015) Expanding the clinical, allelic, and locus heterogeneity of retinal dystrophies. Genet Med
Petrs-Silva H, Linden R (2014) Advances in gene therapy technologies to treat retinitis pigmentosa. Clin Ophthalmol (Auckland, NZ) 8:127
Roman AJ, Schwartz SB, Aleman TS, Cideciyan AV, Chico JD, Windsor EA, Gardner LM, Ying GS, Smilko EE, Maguire MG, Jacobson SG (2005) Quantifying rod photoreceptor-mediated vision in retinal degenerations: dark-adapted thresholds as outcome measures. Exp Eye Res 80:259–272
Roman AJ, Cideciyan AV, Aleman TS, Jacobson SG (2007) Full-field stimulus testing (FST) to quantify visual perception in severely blind candidates for treatment trials. Physiol Meas 28:N51
Smith A, Bainbridge J, Ali R (2012) Gene supplementation therapy for recessive forms of inherited retinal dystrophies. Gene Ther 19:154–161
Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K, Banfi S, Surace EM, Sun J, Acerra C (2013) Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology 120:1283–1291
Thomas CE, Ehrhardt A, Kay MA (2003) Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 4:346–358
Vollrath D, Feng W, Duncan JL, Yasumura D, D’Cruz PM, Chappelow A, Matthes MT, Kay MA, LaVail MM (2001) Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci 98:12584–12589
Wirth T, Parker N, Ylä-Herttuala S (2013) History of gene therapy. Gene 525:162–169
Wright AF, Chakarova CF, El-Aziz MMA, Bhattacharya SS (2010) Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet 11:273–284
Acknowledgments
This study was supported by King Salman Center for Disability Research grant (FSA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
W.W.H. and the University of Florida have a financial interest in the use of AAV therapies, and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work.
Additional information
N. G. Ghazi, E. B. Abboud and S. R. Nowilaty have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
439_2016_1637_MOESM3_ESM.pdf
Figure S1. Cartoon of the vector used in this study. Figure S2. Polyacrylamide electrophoretic gel of GMP AAV2-VMD2-hMERTK vector proteins to test for capsid purity. Lane 9 is the final product and shows that >95% of the protein is composed of the three AAV serotype 2 capsid proteins, thus passing this vector release criterion (PDF 1268 kb)
Rights and permissions
About this article
Cite this article
Ghazi, N.G., Abboud, E.B., Nowilaty, S.R. et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum Genet 135, 327–343 (2016). https://doi.org/10.1007/s00439-016-1637-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-016-1637-y