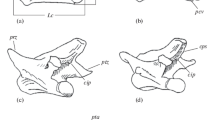
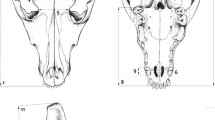
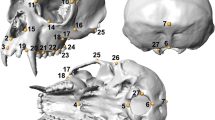
Abstract
The southern short-tailed opossum, Monodelphis dimidiata, is a species known not only for its semelparous life cycle, but also for the extreme sexual dimorphism of adults, where males are not only larger, but also have distinctive morphological characters in their skull. Using geometric morphometrics and a suite of statistical tests, I analyzed the postweaning ontogenetic development of this species in order to evaluate the age-class where sexual dimorphism becomes significant and the amount of change exhibited by both sexes. My results showed that M. dimidiata partly follows the ontogenetic pattern described for didelphids by previous authors. The character that escapes the general pattern is rostral length, which becomes shorter instead of lengthening throughout the development. This change could be related to an increment in the bite force in the anterior part of the dentition. The amount of sexual dimorphism found for this species is larger than the reported previously for other American marsupials, and I also found a higher rate of growth in males at the attaining of sexual maturity. Based on my results and the information available for other didelphids, I can suggest that M. dimidiata males undergo through a process of hypermorphosis, resulting in a peramorphic condition. It is possible that the extreme sexual dimorphism present in this species is related to reproductive success, specially taking into account their semelparous life cycle.





Similar content being viewed by others
References
Abdala F, Flores DA, Giannini NP (2001) Postweaning ontogeny of the skull of Didelphis albiventris. J Mammal 82:190–200
Adams DC, Rohlf FJ, Slice DE (2004) Geometric morphometrics: ten years of progress following the ‘revolution’. Ital J Zool 71:5–16
Astúa D (2010) Cranial sexual dimorphism in New World marsupials and a test of Rensch’s rule in Didelphidae. J Mammal 91:1011–1024
Astúa de Moraes D, Hinghst-Zaher E, Marcus LF, Cerqueira R (2000) A geometric morphometric analysis of cranial and mandibular shape variation of didelphid marsupials. Hystrix 10:115–130
Baladrón AV, Malizia AI, Bó MS, Liébana MS, Bechard MJ (2012) Population dynamics of the southern short-tailed opossum(Monodelphis dimidiata) in the Pampas of Argentina. Aust J Zool 60:238–245
Bergallo HG, Cerqueira R (1994) Reproduction and growth of the opossum Monodelphis domestica (Mammalia, Didelphidae) in northeastern Brazil. J Zool (Lond) 232:551–563
Blanco RE, Jones WW, Milne N (2013) Is the extant southern short-tailed opossum a pigmy sabretooth predator? J Zool 291:100–110
Bookstein FL (1991) Morphometric tools for landmark data: geometry and biology. Cambridge University Press, Cambridge
Bookstein FL (1997) Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med Image Anal 1:225–243
Busch M, Kravetz FO (1991) Diet composition of Monodelphis dimidiata (Marsupialia, Didelphidae). Mammalia 55:619–621
Chemisquy MA, Prevosti FJ (2014) It takes more than large canines to be a sabretooth predator. Mastozool Neotrop 21:27–36
Emerson SB, Bramble DM (1993) Scaling, allometry and skull design. In: Hanken J, Hall BK (eds) The skull, vol 3. The university of Chicago Press, Chicago, pp 384–416
Fischer DA, Cockburn A (2006) The large-male advantage in brown antechinuses: female choice, male dominance, and delayed male death. Behav Ecol 17:164–171
Flores DA, Giannini NP, Abdala F (2003) Cranial ontogeny of Lutreolina crassicaudata (Didelphidae): a comparison with Didelphis albiventris. Acta Theriol 48:1–9
Flores DA, Abdala F, Giannini NP (2010) Cranial ontogeny of Caluromys philander (Didelphidae: Caluromyinae): a qualitative and quantitative approach. J Mammal 91:539–550
Flores DA, Abdala F, Giannini N (2013) Post-weaning cranial ontogeny in two bandicoots (Mammalia, Peramelomorphia, Peramelidae) and comparison with carnivorous marsupials. Zoology 116:372–384
Flores DA, Abdala F, Martin GM, Giannini NP, Martinez JM (2014) Post-weaning cranial growth in shrew opossums (Caenolestidae): a comparison with bandicoots (Peramelidae) and carnivorous marsupials. J Mammal Evol. doi:10.1007/s10914-014-9279-0
García LV (2004) Escaping the Bonferroni iron claw in ecological studies. Oikos 105:657–663
Gardner AL (1973) The systematics of the genus Didelphis (Marsupialia: Didelphidae) in North and Middle America. Spec Publ Mus Texas Tech Univers 4:1–81
Goin FJ, Velázquez C, Scaglia O (1992) Orientación de las crestas cortantes en el molar tribosfénico. Sus implicancias funcionales en didelfoideos (Marsupialia) fósiles y vivientes. Rev Mus La Plata (Nueva Ser) 9:183–198
González EM, Claramunt S (2000) Behaviors of short-tailed opossums, Monodelphis dimidiata. Mammalia 64:271–285
Klingenberg CP (2011) MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour 11:353–357
Klingenberg CP, Barluenga M, Meyer A (2002) Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution 56:1909–1920
Kraaijeveld-Smit FJL, Ward SJ, Temple-Smith PD (2003) Paternity success and the direction of sexual selection in a field population of a semelparous marsupial, Antechinus agilis. Mol Ecol 12:475–484
Maunz M, German RZ (1996) Craniofacial heterochrony and sexual dimorphism in the short tailed opossum (Monodelphis domestica). J Mammal 77:992–1005
Pine RH, Handley CO Jr (2007) Genus Monodelphis. In: Gardner AL (ed) Mammals of South America, vol 1. The University of Chicago Press, Chicago, pp 82–107
Pine RH, Dalby PL, Matson JO (1985) Ecology, postnatal development, morphometrics, and taxanomic status of the short-tailed opossum, Monodelphis dimidiata, an apparently semelparous annual marsupial. Ann Carnegie Mus 54:195–231
Piras P, Salvi D, Ferrara G, Maiorino L, Delfino M, Pedde L, Kotsakis T (2011) The role of post-natal ontogeny in the evolution of phenotypic diversity in Podarcis lizards. J Evol Biol 24:2705–2720
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://wwwR-project.org. Accessed 8 Mar 2015
Radinsky LB (1981) Evolution of skull shape in carnivores 1. Representative modern carnivores. Biol J Linn Soc 15:369–388
Reig OA (1964) Roedores y marsupiales del partido de General Pueyrredón y regiones adyacentes (Provincia de Buenos Aires, Argentina). Publ Mus Mun Cienc Nat, Mar del Plata 1:203–224
Reig OA (1965) Datos sobre la comunidad de pequeños mamíferos de la región costera del Partido de General Pueyrredón y los partidos limítrofes (Prov. de Buenos Aires, Argentina). Physis 25:205–211
Reilly SM, Wiley EO, Meinhardt DJ (1997) An integrative approach to heterochrony: the distinction between interspecific and intraspecific phenomena. Biol J Linn Soc 60:119–143
Rohlf FJ (2008a) TpsDig, ver. 2.12. Department of Ecology and Evolution, State University of New York at Stony Brook, Stony Brook
Rohlf FJ (2008b) TpsRelw, ver. 1.46. Department of Ecology and Evolution, State University of New York at Stony Brook, Stony Brook
Sebastião H, Marroig G (2013) Size and shape in cranial evolution of 2 marsupial genera: Didelphis and Philander (Didelphimorphia, Didelphidae). J Mammal 94:1424–1437
Segura VA (2014) Ontogenia craneana postnatal en cánidos y félidos neotropicales: funcionalidad y patrones evolutivos. Dissertation, Universidad Nacional de La Plata
Sheets HD (2003) IMP-integrated morphometrics package. Department of Physics, Canisius College, Buffalo
Sheets HD, Zelditch ML (2013) Studying ontogenetic trajectories using resampling methods and landmark data. Hystrix 24:67–73
Smith, P (2008) FAUNA Paraguay Handbook of the Mammals of Paraguay Number 26 Monodelphis sorex. www.faunaparaguay.com/monodelphissorex.html. Accessed 15 Dec 2014
Tanner JB, Zelditch ML, Lundrigan BL, Holekamp KE (2010) Ontogenetic change in skull morphology and mechanical advantage in the spotted hyena (Crocuta crocuta). J Morphol 271:353–365
Thompson EN, Biknevicius AR, German RZ (2003) Ontogeny of feeding function in the gray short-tailed opossum Monodelphis domestica: empirical support for the constrained model of jaw biomechanics. J Exp Biol 206:923–932
Ventura J, Pérez-Hernández R, López-Fuster MJ (1998) Morphometric assessment of the Monodelphis brevicaudata group (Didelphimorphia: Didelphidae) in Venezuela. J Mamm 79:104–117
Vilela JF, De Moraes Russo CA, De Oliveira JA (2010) An assessment of morphometric and molecular variation in Monodelphis dimidiata (Wagner, 1847) (Didelphimorphia: Didelphidae). Zootaxa 2646:26–42
Zelditch ML, Swiderski DL, Sheets HD (2012) A practical companion to geometric morphometrics for biologists: running analyses in freely-available software. http://booksite.elsevier.com/9780123869036/. Accessed 14 May 2015
Acknowledgments
Thanks to the following curators for access to the collections under their care and sending additional information: D. Flores (Div. Mastozoología, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”), D. Romero (Área Mastozoología, Museo Municipal de Ciencias Naturales “Lorenzo Scaglia”), S. Bogan (Fundación de Historia Natural Félix de Azara) and I. Olivares (Sección Mastozoología, Museo de La Plata). P. Piras for sending me the R scripts to run the multivariate tests for modeling allometric trajectories. F. Prevosti for the useful discussions and suggestions and critically reading the manuscript. D. Flores for critically reading the manuscript. F. Abdala and an anonymous reviewer made useful suggestions to improve the manuscript. This is a contribution to PICT 2012-0256 from the Agencia Nacional de Promoción Científica y Tecnológica.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Schmidt-Rhaesa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chemisquy, M.A. Peramorphic males and extreme sexual dimorphism in Monodelphis dimidiata (Didelphidae). Zoomorphology 134, 587–599 (2015). https://doi.org/10.1007/s00435-015-0274-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-015-0274-7