Abstract
Background
This retrospective research was designed to investigate the relationship between pT1N0M0 invasive adenocarcinoma (IADC) harboring solid (SOL) and/or micropapillary (MIP) components and its prognosis following lobectomy.
Methods
Clinical data of pT1N0M0 IADC patients were retrospectively collected from Shanghai Chest Hospital. Survival curves were plotted by Kaplan–Meier methods. Multivariable cox regressions were conducted to discover the independent risk factors of recurrence-free survival (RFS) and overall survival (OS), through which nomograms were performed to visualize the risk of recurrences and outcomes in personalized information.
Results
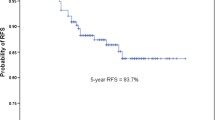
Totally, 1965 patients were enrolled, including 248 harboring SOL/MIP and 1717 not. IADC demonstrated worse 5-year RFS (81.9 vs. 92.2%, p < 0.001) and OS (85.7 vs. 94.4%, p < 0.001) when harboring SOL and/or MIP components. And this status became an independent factor associated with poorer RFS (HR 2.445, 95% CI 1.565–3.821, p < 0.001) and OS (HR 2.139, 95% CI 1.180–3.878, p = 0.012) instead of novel classification of IADC predominant patterns. No difference existed between SOL/MIP predominant and minor patterns. In addition, age > 60, smoking, post-chemotherapy and T1b were all indicating poorer RFS and smoking was also related with worse OS. The c-indexes of nomograms were 0.723 for RFS (95% CI, 0.662–0.784) and 0.703 for OS (95% CI, 0.629–0.777) respectively.
Conclusions
Once the pT1N0M0 IADC harboring SOL/MIP, it strongly indicated the worse clinical recurrence and survival outcome, no matter whether the SOL and/or MIP was predominant. Smoking was correlated with worse prognosis for those patients. Age > 60 and stage T1b also indicated poorer RFS. Whether post-chemotherapy was harmful to pT1N0M0 IADC patients needed further research.



Similar content being viewed by others
Abbreviations
- NSCLC:
-
Non-small-cell lung cancer
- RFS:
-
Recurrence-free survival
- OS:
-
Overall survival
- IADC:
-
Invasive adenocarcinoma
- LEP:
-
Lepidic
- ACN:
-
Acinar
- PAP:
-
Papillary
- SOL:
-
Solid
- MIP:
-
Micropapillary
- AAH:
-
Atypical adenomatous hyperplasia
- AIS:
-
Adenocarcinoma in situ
- MIA:
-
Minimally invasive adenocarcinoma
- PI:
-
Pleural invasion
- LVI:
-
Lymphovascular invasion
- C-index:
-
Concordance index
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Cao Y, Zhu LZ, Jiang MJ et al (2016) Clinical impacts of a micropapillary pattern in lung adenocarcinoma: a review. Onco Targets Ther 9:149–158
Chen Z, Fillmore CM, Hammerman PS et al (2014) Non small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 14(8):535–534
Chen W, Zheng R, Baade PD et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2):115–32
Dai C, Shen J, Ren Y et al (2016) Choice of surgical procedure for patients with NSCLC ≤ 1 cm or 1 to 2 cm among lobectomy, segmentectomy, and wedge resection: a population-based study. J Clin Oncol 34(26):3175–3182
Duan F, Fagerstrom RM et al (2013) Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 368(21):1980–1991
Eng L, Su J, Qiu X et al (2014) Second-hand smoke as a predictor of smoking cessation among lung cancer survivors. J Clin Oncol 32(6):564–570
Ginsberg RJ, Rubinstein LV (1995) Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 60:615–622 (discussion 622–613)
Goldstraw P, Crowley J, Chansky K, International Association for the Study of Lung Cancer International Staging Committee, Participating Institutions et al (2007) The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol 2:706–714
Harrell FE Jr (2001) Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis (Springer series in statistics). Springer, New York
Hung JJ, Wu YC, Chou TY et al (2016) Adjuvant chemotherapy improves the probability of freedom from recurrence in patients with resected stage IB lung adenocarcinoma. Ann Thorac Surg 101(4):1346–1353
Jared D, Christensen CC (2015) Low-dose computed tomographic screening for lung cancer. Clin Chest Med 36:147–160
Little AG, Rusch VW, Bonner JA et al (2005) Patterns of surgical care of lung cancer patients. Ann Thorac Surg 80:2051–2056 (discussion 2056)
Ludwig MS, Goodman M, Miller DL et al (2005) Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 128(3):1545–1550
Maurichi A, Miceli R, Camerini T et al (2014) Prediction of survival in patients with thin melanoma: results from a multi-institution study. J Clin Oncol 32(23):2479–2485
Mollberg NM, Bennette C et al (2014) Lymphovascular invasion as a prognostic indicator in stage I non-small cell lung cancer: a systemic review and meta-analysis. Ann Thorac Surg 97(3):965–971
Morales-Oyarvide V, Mino-Kenudson M (2014) High-grade lung adenocarcinomas with micropapillary and/or solid patterns: a review. Curr Opin Pulm Med 20(4):317–323
Naoki Yanagawa S, Shiono M, Abiko et al (2016) The clinical impact of solid and micropapillary patterns in resected lung adenocarcinoma. J Thorac Oncol 11(11):1976–1983
Nitadori J, Bograd AJ, Kadota K et al (2013) Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2 cm or smaller. J Natl Cancer Inst 105(16):1212–1220
Rami-Porta R, Ball D, Crowley J et al (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2:593–602
Russell PA, Wainer Z, Wright GM et al (2011) Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 6(9):1496–1504
Sica G, Yoshizawa A, Sima CS et al (2010) A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 34(8):1155–1162
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics. CA Cancer J Clin 63:11–30
Travis WD, Garg K, Franklin WA et al (2005) Evolving concepts in the pathology and computed tomography imaging of lung adenocarcinoma and bronchioloalveolar carcinoma. J Clin Oncol 23:3279–3287
Travis WD, Brambilla E, Noguchi M et al (2011) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6(2):244–285
Travis WD, Brambilla E, Nicholson AG et al (2015a) The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10:1243–1260
Travis WD, Brambilla E, Nicholson AG et al (2015b) The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10(9):1243–1260
Travis WD, Asamura H, Bankier AA et al (2016) The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 11(8):1204–1223
Tsao MS, Marguet S, Le Teuff G et al (2015) Subtype classification of lung adenocarcinoma predicts benefit from adjuvant chemotherapy in patients undergoing complete resection. J Clin Oncol 33(30):3439–3446
Waller LL, Weaver KE, Petty WJ et al (2010) Effects of continued tobacco use during treatment of lung cancer. Expert Rev Anticancer Ther 10(10):1569–1575
Yeh YC, Kadota K, Nitadori JI et al (2016) International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification predicts occult lymph node metastasis in clinically mediastinal node-negative lung adenocarcinoma. Eur J Cardiothorac Surg 49(1):e9–e15
Yoshiya T, Mimae T, Tsutani Y et al (2016) Prognostic role of subtype classification in small-sized pathologic N0 invasive lung adenocarcinoma. Ann Thorac Surg 102(5):1668–1673
Zhang J, Wu J, Tan Q et al (2013) Why do pathological stage IA lung adenocarcinomas vary from prognosis? A clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J Thorac Oncol 8(9):1196–1202
Zhang Y, Li J, Wang R et al (2014) The prognostic and predictive value of solid subtype in invasive lung adenocarcinoma. Sci Rep 24(4):7163
Zhao Y, Wang R, Shen X et al (2016) Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol 23(6):2099–2105
Funding
This work was supported by the National Natural Science Foundation of China (81572253, 81330056, 81401891, 81422029 and 81372525).
Author information
Authors and Affiliations
Contributions
HC is the guarantor of the manuscript. YW contributed to conception and study design, acquisition and analysis of data, and writing and revision of the manuscript. DZ contributed to conception and study design, acquisition and analysis of data, and writing and revision of the manuscript. JZ contributed to acquisition of data and writing and revision of the manuscript. QH contributed to acquisition of data and writing and revision of the manuscript. BH contributed to analysis of data and revision of the manuscript. JZ contributed to analysis of data and revision of the manuscript. HZ contributed to analysis of data and revision of the manuscript. HC contributed to conception and study design, analysis of data, and review and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was conducted in line with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and was approved by the Institutional Review Board of Shanghai Chest Hospital.
Informed consent
Written informed consent was obtained from each patient to allow their biological samples to be genetically analyzed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
S1 Fig. 5-year recurrence-free survival (1A) and overall survival (1B) in patients with pT1N0M0 invasive adenocarcinoma underwent lobectomy
. SOL or MIP predominant, solid and/or micropapillary predominant subtypes. SOL or MIP minor, subtypes containing solid and/or micropapillary but not in predominance. SOL or MIP absent, subtypes not containing any solid and/or micropapillary components. Supplementary material 1 (TIF 1396 KB)
Rights and permissions
About this article
Cite this article
Wang, Y., Zheng, D., Zheng, J. et al. Predictors of recurrence and survival of pathological T1N0M0 invasive adenocarcinoma following lobectomy. J Cancer Res Clin Oncol 144, 1015–1023 (2018). https://doi.org/10.1007/s00432-018-2622-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2622-8