Abstract
Purpose
Although androgen-deprivation therapy (ADT) for prostate cancer is initially effective, most tumors eventually recur even during ADT. To predict their prognosis, the Japan Cancer of the Prostate Risk Assessment (J-CAPRA) score was developed. However, there is no validation of this model using data from a single institution. Therefore, in this study, we clarified the oncological outcome of primary ADT and its prognostic factors, as well as validated the J-CAPRA score model in our institution.
Methods
This study included 248 Japanese patients with hormone-naïve prostate cancer who were treated with primary ADT from 1996 through 2012. The oncological outcome and prognostic significance of several clinicopathological factors were analyzed. Also, J-CAPRA risk stratification model was validated in this cohort.
Results
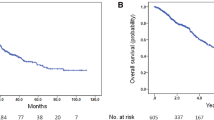
During a median follow-up period of 42.2 months, the median progression-free survival (PFS) and overall survival (OS) were 89.3 and 103.3 months, respectively. Multivariate analysis identified clinical T-stage and M-stage for PFS and cancer-specific survival (CSS) and clinical M-stage for OS as significant predictors. The accuracy of J-CAPRA score model for predicting PFS, CSS, and OS was validated by high c-indices.
Conclusions
This study demonstrated the use of the J-CAPRA score system for predicting PFS, CSS, and OS among Japanese men treated with primary ADT in a single institution.



Similar content being viewed by others
References
Ahmadi H, Daneshmand S (2013) Androgen deprivation therapy: evidence-based management of side effects. BJU Int 111:543–548
Akaza H, Homma Y, Usami M et al (2006) Efficacy of primary hormone therapy for localized or locally advanced prostate cancer: results of a 10-year follow-up. BJU Int 98:573–579
Akaza H, Hinotsu S, Usami M et al (2009) Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer 115:3437–3445
Cancer Registration Committee of the Japanese Urological Association (2005) Clinicopathological statistics on registered prostate cancer patients in Japan: 2000 report from the Japanese Urological Association. Int J Urol 12:46–61
Center MM, Jemal A, Lortet-Tieulent J et al (2012) International variation in prostate cancer incidence and mortality rates. Eur Urol 61:1079–1092
Cooperberg MR, Hinotsu S, Namiki M et al (2009) Risk assessment among prostate cancer patients receiving primary androgen deprivation therapy. J Clin Oncol 27:4306–4313
Epstein JI, Allsbrook WC Jr, Amin MB et al (2005) The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 29:1228–1242
Fujimoto H, Nakanishi H, Miki T et al (2011) Oncological outcomes of the prostate cancer patients registered in 2004: report from the Cancer Registration Committee of the JUA. Int J Urol 18:876–881
Grivas PD, Robins DM, Hussain M (2013) Predicting response to hormonal therapy and survival in men with hormone sensitive metastatic prostate cancer. Crit Rev Oncol Hematol 85:82–93
Harlan LC, Potosky A, Gilliland FD et al (2001) Factors associated with initial therapy for clinically localized prostate cancer: prostate cancer outcomes study. J Natl Cancer Inst 93:1864–1871
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol 65:467–479
Huggins C, Hodges CV (1941) Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1:293–297
International Union Against Cancer (1997) Urologic Tumors. Prostate. In: Sobin LH, Wittekind CH (eds) TNM classification of malignant tumors, 5th edn. Wiley, New York, pp 170–173
Kimura T, Onozawa M, Miyazaki J et al (2014) Prognostic impact of young age on stage IV prostate cancer treated with primary androgen deprivation therapy. Int J Urol 21:578–583
Kitagawa Y, Hinotsu S, Shigehara K et al (2013) Japan Cancer of the Prostate Risk Assessment for combined androgen blockade including bicalutamide: clinical application and validation. Int J Urol 20:708–714
Morote J, Planas J, Salvador C et al (2009) Individual variations of serum testosterone in patients with prostate cancer receiving androgen deprivation therapy. BJU Int 103:332–335
Niraula S, Le LW, Tannock IF (2013) Treatment of prostate cancer with intermittent versus continuous androgen deprivation: a systematic review of randomized trials. J Clin Oncol 31:2029–2036
Park YH, Hwang IS, Jeong CW et al (2009) Prostate specific antigen half-time and prostate specific antigen doubling time as predictors of response to androgen deprivation therapy for metastatic prostate cancer. J Urol 181:2520–2524
Potosky AL, Haque R, Cassidy-Bushrow AE (2014) Effectiveness of primary androgen-deprivation therapy for clinically localized prostate cancer. J Clin Oncol 32:1324–1330
Prostate Cancer Trialists’ Collaborative Group (2000) Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 355:1491–1498
Scher HI, Halabi S, Tannock I et al (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 26:1148–1159
Shiota M (2014) Editorial Comment from Dr Shiota to Prognostic impact of young age on stage IV prostate cancer treated with primary androgen deprivation therapy. Int J Urol 21:583–584
Shiota M, Kashiwagi E, Yokomizo A et al (2013a) Interaction between docetaxel resistance and castration resistance in prostate cancer: implications of Twist1, YB-1, and androgen receptor. Prostate 73:1336–1344
Shiota M, Yokomizo A, Fujimoto N et al (2013b) Castration-resistant prostate cancer: novel therapeutics pre- or post- taxane administration. Curr Cancer Drug Targets 13:444–459
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Sweeney C, Chen YH, Carducci MA et al (2014) Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): An ECOG-led phase III randomized trial. J Clin Oncol 32:5s (suppl; abstr LBA2)
Acknowledgments
We would like to thank Edanz Group Japan for editorial assistance. This work was supported by Kakenhi Grants (24890160 and 25462484) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shiota, M., Yokomizo, A., Takeuchi, A. et al. The oncological outcome and validation of Japan Cancer of the Prostate Risk Assessment score among men treated with primary androgen-deprivation therapy. J Cancer Res Clin Oncol 141, 495–503 (2015). https://doi.org/10.1007/s00432-014-1828-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1828-7