Abstract
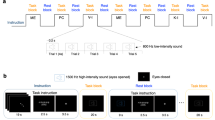
Default in-phase coupling of hand movements needs to be suppressed when temporal coordination is required for out-of-phase bimanual movements. There is lack of knowledge on how the brain overrides these default in-phase movements to enable a required interval of activity between hands. We used a visually cued bimanual temporal coordination (vc-BTC) paradigm with a constant rhythmical time base of 1 s, to test the accuracy of in-phase and out-of-phase (0.1, 0.2,…,0.9) finger tapping. We hypothesized that (1) stronger anatomical and effective interhemispheric connectivity between the hand areas of the primary motor cortex (M1HAND) predict higher temporal offsets between hands in the out-of-phase conditions of the vc-BTC; (2) patients with relapsing-remitting multiple sclerosis (RRMS) and clinically isolated syndrome (CIS) have reduced interhemispheric connectivity and altered between-hand coupling. Anatomical connectivity was determined by fractional anisotropy of callosal hand motor fibers (FA-hCMF). Effective connectivity was probed by short interval interhemispheric inhibition (S-IHI) using paired-coil transcranial magnetic stimulation (TMS). In healthy subjects, higher FA-hCMF and S-IHI correlated with higher temporal offsets between hands in the out-of-phase conditions of the tapping test. FA-hCMF was reduced in patients with RRMS but not in CIS, while S-IHI was reduced in both patient groups. These abnormalities were associated with smaller temporal offsets between hands leading to less deviation from the required phasing in the out-of-phase tapping conditions. Findings provide multiple levels of evidence that callosal anatomical and effective connectivity between the hand areas of the motor cortices play important roles in visually cued bimanual temporal coordination performance.







Similar content being viewed by others
Abbreviations
- CC:
-
Corpus callosum
- CMF:
-
Callosal motor fibers
- CIS:
-
Clinically isolated syndrome
- DTI:
-
Diffusion tensor imaging
- EDSS:
-
Expanded disability status scale
- FA:
-
Fractional anisotropy
- FA-hCMF:
-
Fractional anisotropy of hand callosal motor fibers
- FDI:
-
First dorsal interosseous
- hCMF:
-
Hand callosal motor fibers
- M1HAND :
-
Primary motor cortex hand area
- MEP:
-
Motor evoked potential
- 9-HPT:
-
Nine-hole peg test
- NAWM:
-
Normal appearing with matter
- RMT:
-
Resting motor threshold
- RRMS:
-
Relapsing-remitting multiple sclerosis
- S-IHI:
-
Short interval interhemispheric inhibition
- TMS:
-
Transcranial magnetic stimulation
- vc-BTC:
-
Visually cued bimanual temporal coordination
References
Au Duong MV, Boulanouar K, Audoin B, Treseras S, Ibarrola D, Malikova I, Confort-Gouny S, Celsis P, Pelletier J, Cozzone PJ, Ranjeva JP (2005) Modulation of effective connectivity inside the working memory network in patients at the earliest stage of multiple sclerosis. NeuroImage 24(2):533–538
Audoin B, Au Duong MV, Ranjeva JP, Ibarrola D, Malikova I, Confort-Gouny S, Soulier E, Viout P, Ali-Cherif A, Pelletier J, Cozzone PJ (2005) Magnetic resonance study of the influence of tissue damage and cortical reorganization on PASAT performance at the earliest stage of multiple sclerosis. Hum Brain Mapp 24(3):216–228
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111(3):209–219
Bonzano L, Tacchino A, Roccatagliata L, Abbruzzese G, Mancardi GL, Bove M (2008) Callosal contributions to simultaneous bimanual finger movements. J Neurosci 28(12):3227–3233
Bonzano L, Sormani MP, Tacchino A, Abate L, Lapucci C, Mancardi GL, Uccelli A, Bove M (2013) Quantitative assessment of finger motor impairment in multiple sclerosis. PLoS One 8(5):e65225. doi:10.1371/journal.pone.0065225
Bonzano L, Tacchino A, Brichetto G, Roccatagliata L, Dessypris A, Feraco P, Lopes De Carvalho ML, Battaglia MA, Mancardi GL, Bove M (2014) Upper limb motor rehabilitation impacts white matter microstructure in multiple sclerosis. NeuroImage 90:107–116. doi:10.1016/j.neuroimage.2013.12.025
Byblow WD, Carson RG, Goodman D (1994) Expressions of asymmetries and anchoring in bimanual coordination. Hum Mov Sci 13(1):3–28. doi:10.1016/0167-9457(94)90027-2
Caeyenberghs K, Leemans A, Coxon J, Leunissen I, Drijkoningen D, Geurts M, Gooijers J, Michiels K, Sunaert S, Swinnen SP (2011) Bimanual coordination and corpus callosum microstructure in young adults with traumatic brain injury: a diffusion tensor imaging study. J Neurotrauma 28(6):897–913. doi:10.1089/neu.2010.1721
Caille S, Sauerwein HC, Schiavetto A, Villemure JG, Lassonde M (2005) Sensory and motor interhemispheric integration after section of different portions of the anterior corpus callosum in nonepileptic patients. Neurosurgery 57(1):50–59
Eliassen JC, Baynes K, Gazzaniga MS (1999) Direction information coordinated via the posterior third of the corpus callosum during bimanual movements. Exp Brain Res 128(4):573–577
Eliassen JC, Baynes K, Gazzaniga MS (2000) Anterior and posterior callosal contributions to simultaneous bimanual movements of the hands and fingers. Brain 123(Pt 12):2501–2511
Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM (2000a) Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol 47(3):391–395
Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM (2000b) Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain 123(Pt 9):1845–1849
Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD (1992) Interhemispheric inhibition of the human motor cortex. J Physiol (Lond) 453:525–546
Fling BW, Seidler RD (2012) Fundamental differences in callosal structure, neurophysiologic function, and bimanual control in young and older adults. Cereb Cortex 22(11):2643–2652. doi:10.1093/cercor/bhr349
Fling BW, Walsh CM, Bangert AS, Reuter-Lorenz PA, Welsh RC, Seidler RD (2011) Differential callosal contributions to bimanual control in young and older adults. J Cogn Neurosci 23(9):2171–2185. doi:10.1162/jocn.2010.21600
Fling BW, Benson BL, Seidler RD (2013) Transcallosal sensorimotor fiber tract structure-function relationships. Hum Brain Mapp 34(2):384–395. doi:10.1002/hbm.21437
Franz EA, Eliassen JC, Ivry RB, Gazzaniga MS (1996) Dissociation of spatial and temporal coupling in the bimanual movements of callosotomy patients. Psychol Sci 7(5):306–310. doi:10.1111/J.1467-9280.1996.Tb00379.X
Friston KJ, Frith CD, Liddle PF, Frackowiak RS (1993) Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab 13(1):5–14
Gamboa OL, Tagliazucchi E, von Wegner F, Jurcoane A, Wahl M, Laufs H, Ziemann U (2014) Working memory performance of early MS patients correlates inversely with modularity increases in resting state functional connectivity networks. NeuroImage 94C:385–395. doi:10.1016/j.neuroimage.2013.12.008
Ge Y, Law M, Johnson G, Herbert J, Babb JS, Mannon LJ, Grossman RI (2004) Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J Magn Reson Imaging 20(1):1–7
Giorgio A, Portaccio E, Stromillo ML, Marino S, Zipoli V, Battaglini M, Blandino A, Bartolozzi ML, Siracusa G, Amato MP, De Stefano N (2010) Cortical functional reorganization and its relationship with brain structural damage in patients with benign multiple sclerosis. Mult Scler 16(11):1326–1334
Gooijers J, Swinnen SP (2014) Interactions between brain structure and behavior: the corpus callosum and bimanual coordination. Neurosci Biobehav Rev 43:1–19. doi:10.1016/J.Neubiorev.03.008
Gooijers J, Caeyenberghs K, Sisti HM, Geurts M, Heitger MH, Leemans A, Swinnen SP (2013) Diffusion tensor imaging metrics of the corpus callosum in relation to bimanual coordination: effect of task complexity and sensory feedback. Hum Brain Mapp 34(1):241–252. doi:10.1002/hbm.21429
Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, Thickbroom GW, Rossini PM, Ziemann U, Valls-Sole J, Siebner HR (2012) A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 123(5):858–882
Harrison DM, Caffo BS, Shiee N, Farrell JA, Bazin PL, Farrell SK, Ratchford JN, Calabresi PA, Reich DS (2011) Longitudinal changes in diffusion tensor-based quantitative MRI in multiple sclerosis. Neurology 76(2):179–186
Hofer S, Frahm J (2006) Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage 32(3):989–994
Hübers A, Orekhov Y, Ziemann U (2008) Interhemispheric motor inhibition: its role in controlling electromyographic mirror activity. Eur J Neurosci 28:364–371
Jeeves MA, Silver PH, Jacobson I (1988) Bimanual co-ordination in callosal agenesis and partial commissurotomy. Neuropsychologia 26(6):833–850
Jirsa VK, Fuchs A, Kelso JA (1998) Connecting cortical and behavioral dynamics: bimanual coordination. Neural Comput 10(8):2019–2045
Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T (2007) Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. NeuroImage 36(Suppl 2):T16–T21
Kelso JA (1984) Phase transitions and critical behavior in human bimanual coordination. Am J Physiol 246(6 Pt 2):R1000–R1004
Kelso JA, Holt KG, Rubin P, Kugler PN (1981) Patterns of human interlimb coordination emerge from the properties of non-linear, limit cycle oscillatory processes: theory and data. J Mot Behav 13(4):226–261
Kennerley SW, Diedrichsen J, Hazeltine E, Semjen A, Ivry RB (2002) Callosotomy patients exhibit temporal uncoupling during continuous bimanual movements. Nat Neurosci 5(4):376–381
Kovacs AJ, Shea CH (2011) The learning of 90 degrees continuous relative phase with and without Lissajous feedback: external and internally generated bimanual coordination. Acta Psychol 136(3):311–320. doi:10.1016/j.actpsy.2010.12.004
Larson EB, Burnison DS, Brown WS (2002) Callosal function in multiple sclerosis: bimanual motor coordination. Cortex 38(2):201–214
Lazar M, Weinstein DM, Tsuruda JS, Hasan KM, Arfanakis K, Meyerand ME, Badie B, Rowley HA, Haughton V, Field A, Alexander AL (2003) White matter tractography using diffusion tensor deflection. Hum Brain Mapp 18(4):306–321
Mathiowetz V, Volland G, Kashman N, Weber K (1985) Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther 39(6):386–391
Meyer BU, Röricht S, Gräfin von Einsiedel H, Kruggel F, Weindl A (1995) Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 118(Pt 2):429–440
Mori S, van Zijl PC (2002) Fiber tracking: principles and strategies—a technical review. NMR Biomed 15(7–8):468–480
Muetzel RL, Collins PF, Mueller BA, M Schissel A, Lim KO, Luciana M (2008) The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescents. NeuroImage 39(4):1918–1925. doi:10.1016/j.neuroimage.2007.10.018
Nelson AJ, Hoque T, Gunraj C, Ni Z, Chen R (2009) Bi-directional interhemispheric inhibition during unimanual sustained contractions. BMC Neurosci 10(1):31
Netz J, Ziemann U, Hömberg V (1995) Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res 104(3):527–533
Nimsky C, Ganslandt O, Fahlbusch R (2006) Implementation of fiber tract navigation. Neurosurgery 58(4 Suppl 2):ONS-292–ONS-303 (discussion ONS-303–ONS-294)
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Pelletier J, Suchet L, Witjas T, Habib M, Guttmann CR, Salamon G, Lyon-Caen O, Cherif AA (2001) A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing-remitting multiple sclerosis. Arch Neurol 58(1):105–111
Penner IK, Rausch M, Kappos L, Opwis K, Radu EW (2003) Analysis of impairment related functional architecture in MS patients during performance of different attention tasks. J Neurol 250(4):461–472
Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996) Diffusion tensor MR imaging of the human brain. Radiology 201(3):637–648
Pluim JP, Maintz JB, Viergever MA (2003) Mutual-information-based registration of medical images: a survey. IEEE Trans Med Imaging 22(8):986–1004. doi:10.1109/TMI.2003.815867
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol 69(2):292–302
Preilowski BF (1972) Possible contribution of the anterior forebrain commissures to bilateral motor coordination. Neuropsychologia 10(3):267–277
Pulizzi A, Rovaris M, Judica E, Sormani MP, Martinelli V, Comi G, Filippi M (2007) Determinants of disability in multiple sclerosis at various disease stages: a multiparametric magnetic resonance study. Arch Neurol 64(8):1163–1168
Ranjeva JP, Pelletier J, Confort-Gouny S, Ibarrola D, Audoin B, Le Fur Y, Viout P, Cherif AA, Cozzone PJ (2003) MRI/MRS of corpus callosum in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler 9(6):554–565
Rocca MA, Mezzapesa DM, Ghezzi A, Falini A, Martinelli V, Scotti G, Comi G, Filippi M (2005) A widespread pattern of cortical activations in patients at presentation with clinically isolated symptoms is associated with evolution to definite multiple sclerosis. Am J Neuroradiol 26(5):1136–1139
Rocca MA, Pagani E, Absinta M, Valsasina P, Falini A, Scotti G, Comi G, Filippi M (2007) Altered functional and structural connectivities in patients with MS: a 3-T study. Neurology 69(23):2136–2145
Rocca MA, Valsasina P, Absinta M, Riccitelli G, Rodegher ME, Misci P, Rossi P, Falini A, Comi G, Filippi M (2010) Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology 74(16):1252–1259
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120(12):2008–2039
Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M (1994) Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res 102(2):227–243
Ruohonen J, Karhu J (2010) Navigated transcranial magnetic stimulation. Clin Neurophysiol 40(1):7–17. doi:10.1016/j.neucli.2010.01.006
Seitz R, Kleiser R, Butefisch C, Jorgens S, Neuhaus O, Hartung HP, Wittsack HJ, Sturm V, Hermann M (2004) Bimanual recoupling by visual cueing in callosal disconnection. Neurocase 10(4):316–325
Serbruyns L, Gooijers J, Caeyenberghs K, Meesen RL, Cuypers K, Sisti HM, Leemans A, Swinnen SP (2013) Bimanual motor deficits in older adults predicted by diffusion tensor imaging metrics of corpus callosum subregions. Brain Struct Funct. doi:10.1007/s00429-013-0654-z
Serrien DJ, Nirkko AC, Wiesendanger M (2001) Role of the corpus callosum in bimanual coordination: a comparison of patients with congenital and acquired callosal damage. Eur J Neurosci 14:1897–1905
Sigal T, Shmuel M, Mark D, Gil H, Anat A (2012) Diffusion tensor imaging of corpus callosum integrity in multiple sclerosis: correlation with disease variables. J Neuroimaging 22(1):33–37. doi:10.1111/j.1552-6569.2010.00556.x
Sisti HM, Geurts M, Gooijers J, Heitger MH, Caeyenberghs K, Beets IA, Serbruyns L, Leemans A, Swinnen SP (2012) Microstructural organization of corpus callosum projections to prefrontal cortex predicts bimanual motor learning. Learn Mem 19(8):351–357. doi:10.1101/lm.026534.112
Staffen W, Mair A, Zauner H, Unterrainer J, Niederhofer H, Kutzelnigg A, Ritter S, Golaszewski S, Iglseder B, Ladurner G (2002) Cognitive function and fMRI in patients with multiple sclerosis: evidence for compensatory cortical activation during an attention task. Brain 125(Pt 6):1275–1282
Stephan KM, Binkofski F, Halsband U, Dohle C, Wunderlich G, Schnitzler A, Tass P, Posse S, Herzog H, Sturm V, Zilles K, Seitz RJ, Freund H-J (1999) The role of ventral medial wall motor areas in bimanual co-ordination. Brain 122(2):351–368
Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A (2001) Equivalent disruption of regional white matter microstructure in ageing healthy men and women. NeuroReport 12(1):99–104
Swinnen SP (2002) Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci 3(5):348–359
Tuller B, Kelso JA (1989) Environmentally-specified patterns of movement coordination in normal and split-brain subjects. Exp Brain Res 75(2):306–316
Vollmann H, Ragert P, Conde V, Villringer A, Classen J, Witte OW, Steele CJ (2014) Instrument specific use-dependent plasticity shapes the anatomical properties of the corpus callosum: a comparison between musicians and non-musicians. Front Behav Neurosci 8:245. doi:10.3389/fnbeh.2014.00245
Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U (2007) Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci 27(45):12132–12138
Wahl M, Hübers A, Lauterbach-Soon B, Hattingen E, Jung P, Cohen LG, Ziemann U (2011) Motor callosal disconnection in early relapsing-remitting multiple sclerosis. Hum Brain Mapp 32(6):846–855
Weinstein D, Kindlmann G, Lundberg E (1999) Tensorlines. Advection-diffusion based propagation through diffusion tensor fields. Paper presented at the proceedings of the conference on visualization
Weiss C, Nettekoven C, Rehme AK, Neuschmelting V, Eisenbeis A, Goldbrunner R, Grefkes C (2013) Mapping the hand, foot and face representations in the primary motor cortex—retest reliability of neuronavigated TMS versus functional MRI. NeuroImage 66:531–542. doi:10.1016/j.neuroimage.2012.10.046
Wittstock M, Grossmann A, Kunesch E, Walter U, Benecke R, Wolters A (2010) Altered callosal function in cerebral microangiopathy. J Neurol 257(4):590–597. doi:10.1007/s00415-009-5379-9
Yamanishi J, Kawato M, Suzuki R (1979) Studies on human finger tapping neural networks by phase transition curves. Biol Cybern 33(4):199–208
Yamanishi J, Kawato M, Suzuki R (1980) Two coupled oscillators as a model for the coordinated finger tapping by both hands. Biol Cybern 37(4):219–225
Yu CS, Lin FC, Liu Y, Duan Y, Lei H, Li KC (2008) Histogram analysis of diffusion measures in clinically isolated syndromes and relapsing-remitting multiple sclerosis. Eur J Radiol 68(2):328–334
Zaitsev M, Hennig J, Speck O (2004) Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust, and flexible method for echo-planar imaging distortion correction. Magn Reson Med 52(5):1156–1166
Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM (2006) Functional anatomy of interhemispheric cortical connections in the human brain. J Anat 209(3):311–320
Zito G, Luders E, Tomasevic L, Lupoi D, Toga AW, Thompson PM, Rossini PM, Filippi MM, Tecchio F (2014) Inter-hemispheric functional connectivity changes with corpus callosum morphology in multiple sclerosis. Neuroscience 266:47–55. doi:10.1016/j.neuroscience.2014.01.039
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they do not have any conflicts of interest.
Funding
This study was supported by a grant from TEVA Pharma GmbH.
Rights and permissions
About this article
Cite this article
Wahl, M., Lauterbach-Soon, B., Hattingen, E. et al. Callosal anatomical and effective connectivity between primary motor cortices predicts visually cued bimanual temporal coordination performance. Brain Struct Funct 221, 3427–3443 (2016). https://doi.org/10.1007/s00429-015-1110-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-015-1110-z