Abstract
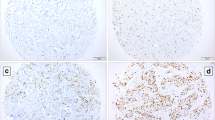
Neuroendocrine tumors (NET) are routinely graded and staged to judge prognosis. Proliferation index using MIB1 staining has been introduced to assess grading. There are vivid discussions on cutoff definitions, automated counting, and interobserver variability. However, no data exist regarding interlaboratory reproducibility for low proliferation indices which are of importance to discriminate between G1 and G2 NET. We performed MIB1 staining in three different university hospital-based pathology laboratories on a tissue micro array (TMA) of a well-characterized patient cohort, containing pancreatic NET of 61 patients. To calculate the proliferation index, number of positive tumor nuclei was divided by the total number of tumor nuclei. Labeling index was compared to mitotic counts in whole tissue sections and to clinical outcome. Linear regression analysis, intraclass comparison, and log-rank analysis were performed. Intraclass correlation showed moderate-to-fair agreement. Especially low proliferating tumors were affected by interlaboratory differences. Log-rank analysis was performed for each lab and resulted in three different cutoffs (5.0, 3.0, and 0.5 %). Every calculated cutoff stratified the patient cohort to a significant extent for the underlying stain (p < 0.001, <0.001, and <0.001) but showed no or lesser significance when applied to the other stains. Significant and relevant interlab differences for MIB1 exist. Since the MIB1 proliferation index influences grading, local cutoffs or external standardization should urgently be introduced to achieve reliability and reproducibility.



Similar content being viewed by others
References
Couvelard A, O'Toole D, Turley H, Leek R, Sauvanet A, Degott C, Ruszniewski P, Belghiti J, Harris AL, Gatter K, Pezzella F (2005) Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer 92:94–101. doi:10.1038/sj.bjc.6602245
Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, Klimstra DS (2002) Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol 20:2633–2642
La Rosa S, Sessa F, Capella C, Riva C, Leone BE, Klersy C, Rindi G, Solcia E (1996) Prognostic criteria in nonfunctioning pancreatic endocrine tumours. Virchows Arch 429:323–333
Pelosi G, Bresaola E, Bogina G, Pasini F, Rodella S, Castelli P, Iacono C, Serio G, Zamboni G (1996) Endocrine tumors of the pancreas: Ki-67 immunoreactivity on paraffin sections is an independent predictor for malignancy: a comparative study with proliferating-cell nuclear antigen and progesterone receptor protein immunostaining, mitotic index, and other clinicopathologic variables. Hum Pathol 27:1124–1134
Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B (2008) Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res 14:7798–7803. doi:10.1158/1078-0432.CCR-08-0734
Rindi G, Kloppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, Korner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B (2006) TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 449:395–401
Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, Panzuto F, Pederzoli P, Delle Fave G, Falconi M (2010) Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol 23:824–833. doi:10.1038/modpathol.2010.58
Heitz P, Komminoth P, Perren A, Klimstra DS, Dayal Y, Bordi C, Lechago J, Centeno BA, Klöppel G (2004) WHO classification of tumours, Pathology and genetics of tumours of endocrine organs. In: De Lellis R, Heitz P, Lloyd R, Eng C (eds) Tumours of the endocrine pancreas (chapter 4). IARC, Lyon
Pavel M, Baudin E, Couvelard A, Krenning E, Oberg K, Steinmuller T, Anlauf M, Wiedenmann B, Salazar R, Conference BC (2012) ENETS consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 95:157–176. doi:10.1159/000335597
Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J (1992) Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 168:357–363. doi:10.1002/path.1711680404
Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13–20
Jamali M, Chetty R (2008) Predicting prognosis in gastroentero-pancreatic neuroendocrine tumors: an overview and the value of Ki-67 immunostaining. Endocr Pathol 19:282–288
Clarke MR, Baker EE, Weyant RJ, Hill L, Carty SE (1997) Proliferative activity in pancreatic endocrine tumors: association with function, metastases, and survival. Endocr Pathol 8:181–187
Panzuto F, Boninsegna L, Fazio N, Campana D, Pia Brizzi M, Capurso G, Scarpa A, De Braud F, Dogliotti L, Tomassetti P, Delle Fave G, Falconi M (2011) Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol 29:2372–2377
Klimstra DS, Modlin IR, Adsay NV, Chetty R, Deshpande V, Gonen M, Jensen RT, Kidd M, Kulke MH, Lloyd RV, Moran C, Moss SF, Oberg K, O'Toole D, Rindi G, Robert ME, Suster S, Tang LH, Tzen CY, Washington MK, Wiedenmann B, Yao J (2010) Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol 34:300–313
Lowe K, Khithani A, Liu E, Winston T, Christian D, Saad J, Jeyarajah DR (2012) Ki-67 labeling: a more sensitive indicator of malignant phenotype than mitotic count or tumor size? J Surg Oncol 106:724–727
Rindi G, Klersy C, Inzani F, Fellegara G, Ampollini L, Ardizzoni A, Campanini N, Carbognani P, De Pas TM, Galetta D, Granone PL, Righi L, Rusca M, Spaggiari L, Tiseo M, Viale G, Volante M, Papotti M, Pelosi G (2014) Grading the neuroendocrine tumors of the lung: an evidence-based proposal. Endocr Relat Cancer 21:1–16. doi:10.1530/ERC-13-0246
Goodell PP, Krasinskas AM, Davison JM, Hartman DJ (2012) Comparison of methods for proliferative index analysis for grading pancreatic well-differentiated neuroendocrine tumors. Am J Clin Pathol 137:576–582. doi:10.1309/AJCP92UCXPJMMSDU
Reid MD, Bagci P, Ohike N, Saka B, Erbarut Seven I, Dursun N, Balci S, Gucer H, Jang KT, Tajiri T, Basturk O, Kong SY, Goodman M, Akkas G, Adsay V (2015) Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies. Mod Pathol 28:686–694. doi:10.1038/modpathol.2014.156
Tang LH, Gonen M, Hedvat C, Modlin IM, Klimstra DS (2012) Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. Am J Surg Pathol 36:1761–1770. doi:10.1097/PAS.0b013e318263207c
Varga Z, Diebold J, Dommann-Scherrer C, Frick H, Kaup D, Noske A, Obermann E, Ohlschlegel C, Padberg B, Rakozy C, Sancho Oliver S, Schobinger-Clement S, Schreiber-Facklam H, Singer G, Tapia C, Wagner U, Mastropasqua MG, Viale G, Lehr HA (2012) How reliable is Ki-67 immunohistochemistry in grade 2 breast carcinomas? A QA study of the Swiss Working Group of Breast- and Gynecopathologists. PLoS One 7:e37379. doi:10.1371/journal.pone.0037379
Hsu CY, Ho DM, Yang CF, Chiang H (2003) Interobserver reproducibility of MIB-1 labeling index in astrocytic tumors using different counting methods. Mod Pathol 16:951–957. doi:10.1097/01.MP.0000084631.64279.BC
Mengel M, von Wasielewski R, Wiese B, Rudiger T, Muller-Hermelink HK, Kreipe H (2002) Inter-laboratory and inter-observer reproducibility of immunohistochemical assessment of the Ki-67 labelling index in a large multi-centre trial. J Pathol 198:292–299
Schmitt AM, Anlauf M, Rousson V, Schmid S, Kofler A, Riniker F, Bauersfeld J, Barghorn A, Probst-Hensch NM, Moch H, Heitz PU, Kloeppel G, Komminoth P, Perren A (2007) WHO 2004 criteria and CK19 are reliable prognostic markers in pancreatic endocrine tumors. Am J Surg Pathol 31:1677–1682. doi:10.1097/PAS.0b013e31805f675d
Voss SM, Riley MP, Lokhandwala PM, Wang M, Yang Z (2015) Mitotic count by phosphohistone H3 immunohistochemical staining predicts survival and improves interobserver reproducibility in well-differentiated neuroendocrine tumors of the pancreas. Am J Surg Pathol 39:13–24. doi:10.1097/PAS.0000000000000341
Klimstra DSCC, Arnold R (2010) Neuroendocrine neoplasms of the pancreas. IARC, Lyon
Kloppel G, Perren A, Heitz PU (2004) The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci 1014:13–27
Muller MM (2000) Implementation of reference systems in laboratory medicine. Clin Chem 46:1907–1909
Verderio P, Dittadi R, Marubini E, Pizzamiglio S, Gion M, De Apollonia L, Paradiso A, Italian Network for Quality Assessment of Tumor Biomarkers Group (2007) An Italian program of external quality control for chromogranin A (CgA) assay: performance evaluation of CgA determination. Clin Chem Lab Med 45:1244–1250. doi:10.1515/CCLM.2007.251
Yang Z, Tang LH, Klimstra DS (2011) Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: implications for prognostic stratification. Am J Surg Pathol 35:853–860. doi:10.1097/PAS.0b013e31821a0696
Bai Y, Tolles J, Cheng H, Siddiqui S, Gopinath A, Pectasides E, Camp RL, Rimm DL, Molinaro AM (2011) Quantitative assessment shows loss of antigenic epitopes as a function of pre-analytic variables. Lab Investig 91:1253–1261. doi:10.1038/labinvest.2011.75
Benini E, Rao S, Daidone MG, Pilotti S, Silvestrini R (1997) Immunoreactivity to MIB-1 in breast cancer: methodological assessment and comparison with other proliferation indices. Cell Prolif 30:107–115
Srinivasan M, Sedmak D, Jewell S (2002) Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol 161:1961–1971. doi:10.1016/S0002-9440(10)64472-0
Frost AR, Sparks D, Grizzle WE (2000) Methods of antigen recovery vary in their usefulness in unmasking specific antigens in immunohistochemistry. Appl Immunohistochem Mol Morphol 8:236–243
Heuschmid M, Hofmockel G, Dexler B, Dammrich J, Bassukas ID (2002) Different antigen unmasking techniques lead to significant differences in immunohistochemical staining of Ki-67 (Mib-1) in renal cell carcinomas. Oncol Rep 9:19–22
Munakata S, Hendricks JB (1993) Effect of fixation time and microwave oven heating time on retrieval of the Ki-67 antigen from paraffin-embedded tissue. J Histochem Cytochem 41:1241–1246
McCormick D, Yu C, Hobbs C, Hall PA (1993) The relevance of antibody concentration to the immunohistological quantification of cell proliferation-associated antigens. Histopathology 22:543–547
Meyer JS, Alvarez C, Milikowski C, Olson N, Russo I, Russo J, Glass A, Zehnbauer BA, Lister K, Parwaresch R, Cooperative Breast Cancer Tissue Resource (2005) Breast carcinoma malignancy grading by Bloom-Richardson system vs proliferation index: reproducibility of grade and advantages of proliferation index. Mod Pathol 18:1067–1078. doi:10.1038/modpathol.3800388
Vereecken P, Laporte M, Heenen M (2007) Significance of cell kinetic parameters in the prognosis of malignant melanoma: a review. J Cutan Pathol 34:139–145. doi:10.1111/j.1600-0560.2006.00588.x
Grimaldi F, Muser D, Beltrami CA, Machin P, Morelli A, Pizzolitto S, Talmassons G, Marciello F, Colao AA, Monaco R, Monaco G, Faggiano A (2011) Partitioning of bronchopulmonary carcinoids in two different prognostic categories by ki-67 score. Front Endocrinol 2:20. doi:10.3389/fendo.2011.00020
Zahel T, Krysa S, Herpel E, Stenzinger A, Goeppert B, Schirmacher P, Hoffmann H, Schnabel PA, Warth A (2012) Phenotyping of pulmonary carcinoids and a Ki-67-based grading approach. Virchows Arch 460:299–308
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blank, A., Wehweck, L., Marinoni, I. et al. Interlaboratory variability of MIB1 staining in well-differentiated pancreatic neuroendocrine tumors. Virchows Arch 467, 543–550 (2015). https://doi.org/10.1007/s00428-015-1843-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1843-3