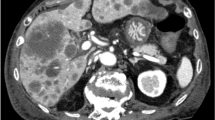
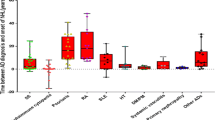
Abstract
We retrospectively analyzed in 54 consecutively enrolled Japanese patients with rheumatoid arthritis (RA) and lymphoproliferative disease (LPD) relevant clinicopathological characteristics, in particular paying attention to treatment with methotrexate (MTX). Between the 28 patients treated with MTX (MTX-treated group) and the 26 who were not (non-MTX group), there was no difference in age, interval between onset of RA and LPD, and lymphoma stage. Immunohistochemical analysis showed that in the MTX-treated group, 15 (53 %) patients had mature B-cell LPD, eight (29 %) mature T/NK-cell LPD, and five (18 %) had Hodgkin lymphoma. In the non-MTX group, 22 (84 %) had mature B-cell LPD, 2 (8 %) had mature T/NK-cell LPD, and 2 (8 %) had Hodgkin lymphoma. The frequency of mature T/NK-cell LPD was significantly higher in the MTX-treated group (p < 0.05). Of the eight patients in the MTX-treated group with mature T/NK-cell LPD, two had large granular lymphocytic leukemia and the other six had a variety of different histological types with frequent CD8 but not CD56 expression. Epstein–Barr virus (EBV) infection was significantly higher in the MTX-treated group (p < 0.05); evidence of latent type II EBV infection was found in four of the eight patients with mature T/NK-cell LPD. Withdrawal of MTX led to complete remission in seven patients with mature T/NK-cell LPD irrespective of EBV infection. Our findings highlight that mature T/NK-cell LPD is a frequent complication in RA patients treated with MTX. EBV infection may play a role in the pathogenesis of T/NK-cell LPD, as well as B-cell LPD and Hodgkin lymphoma in MTX-treated RA patients.




Similar content being viewed by others
References
Wolfe F, Michaud K (2007) Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum 56:2886–2895
Wong AK, Kerkoutian S, Said J, Rashidi H, Pullarkat ST (2011) Risk of lymphoma in patients receiving antitumor necrosis factor therapy: a meta-analysis of published randomized controlled studies. Clin Rheumatol 31:631–636
Yamada T, Nakajima A, Inoue E et al (2011) Incidence of malignancy in Japanese patients with rheumatoid arthritis. Rheumatol Int 31:1487–1492
Kamel OW, van de Rijn M, Weiss LM et al (1993) Brief report: reversible lymphomas associated with Epstein-Barr virus occurring during methotrexate therapy for rheumatoid arthritis and dermatomyositis. N Engl J Med 328:1317–1321
Salloum E, Cooper DL, Howe G et al (1996) Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol 14:1943–1949
Rizzi R, Curci P, Delia M et al (2009) Spontaneous remission of "methotrexate-associated lymphoproliferative disorders" after discontinuation of immunosuppressive treatment for autoimmune disease. Review of the literature. Med Oncol 26:1–9
Harris NL, Swerdlow S (2001) Methotrexate-associatred lymphoprolieferative disorders. In: Jaffe ES, Harris NL, Stein H, Vardiman J (eds) Pathology and genetics, tumors of haematopoietic and lymphoid tissues, World Health Organization classification of tumors. IARC Press, Lyon, pp 270–271
Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM (2002) Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum 46:3151–3158
Gaulard P, Swerdlow SH, Harris NL, Jaffe ES, Sundstrom C (2008) Other iatrogenic immunodeficiency-associatred lymphoprolieferative disorders. In: Swerdlow SH, Campo E, Harris NL et al (eds) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC press, Lyon, pp 350–351
Mariette X, Cazals-Hatem D, Warszawki J, Liote F, Balandraud N, Sibilia J (2002) Lymphomas in rheumatoid arthritis patients treated with methotrexate: a 3-year prospective study in France. Blood 99:3909–3915
Lunemann JD, Frey O, Eidner T et al (2008) Increased frequency of EBV-specific effector memory CD8+ T cells correlates with higher viral load in rheumatoid arthritis. J Immunol 181:991–1000
Shah A, Diehl LF, St Clair EW (2009) T cell large granular lymphocyte leukemia associated with rheumatoid arthritis and neutropenia. Clin Immunol 132:145–152
Arnett FC, Edworthy SM, Bloch DA et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Howe JG, Steitz JA (1986) Localization of Epstein-Barr virus small RNAs by in situ hybridization. Proc Natl Acad Sci U S A 83:9006–9010
Gibson SE, Swerdlow SH, Craig FE et al (2011) EBV-postive extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue in the posttransplant setting: a distinct type of posttransplant lymphoproliferative disorder? Am J Surg Pathol 35:807–815
Fox CP, Shannon-Lowe C, Rowe M (2011) Deciphering the role of Epstein-Barr virus in the pathogenesis of T and NK cell lymphoproliferations. Herpesviridae. doi:10.1186/2042-4280-2-8
McKelvey EM, Gottlieb JA, Wilson HE et al (1976) Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer 38:1484–1493
Bonadonna G, Zucali R, Monfardini S, De Lena M, Uslenghi C (1975) Combination chemotherapy of Hodgkin's disease with adriamycin, bleomycin, vinblastine, and imidazole carboxamide versus MOPP. Cancer 36:252–259
Kojima M, Itoh H, Hirabayashi K et al (2006) Methotrexate-associated lymphoproliferative disorders. A clinicopathological study of 13 Japanese cases. Pathol Res Pract 202:679–685
Nemoto Y, Taniguchi A, Kamioka M et al (2010) Epstein-Barr virus-infected subcutaneous panniculitis-like T-cell lymphoma associated with methotrexate treatment. Int J Hematol 92:364–368
Hatanaka K, Nakamura N, Kojima M et al (2010) Methotrexate-associated lymphoproliferative disorders mimicking angioimmunoblastic T-cell lymphoma. Pathol Res Pract 206:9–13
Hatachi S, Kunitomi A, Aozasa K, Yagita M (2010) CD8(+) T-cell lymphoproliferative disorder associated with Epstein-Barr virus in a patient with rheumatoid arthritis during methotrexate therapy. Mod Rheumatol 20:500–505
Parker SR, Solomon AR, Lane JE (2008) A report of Epstein-Barr virus-positive primary cutaneous natural killer-/T-cell lymphoma. J Am Acad Dermatol 59:157–161
Kono H, Inokuma S, Matsuzaki Y et al (1999) Two cases of methotrexate induced lymphomas in rheumatoid arthritis: an association with increased serum IgE. J Rheumatol 26:2249–2253
Prochorec-Sobieszek M, Rymkiewicz G, Makuch-Lasica H et al (2008) Characteristics of T-cell large granular lymphocyte proliferations associated with neutropenia and inflammatory arthropathy. Arthritis Res Ther. doi:10.1186/ar2424
Menke DM, Griesser H, Moder KG et al (2000) Lymphomas in patients with connective tissue disease. Comparison of p53 protein expression and latent EBV infection in patients immunosuppressed and not immunosuppressed with methotrexate. Am J Clin Pathol 113:212–218
Feng WH, Cohen JI, Fischer S et al (2004) Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. J Natl Cancer Inst 96:1691–1702
Le Goff P, Chicault P, Saraux A, Baron D, Valls-Bellec I, Leroy JP (1998) Lymphoma with regression after methotrexate withdrawal in a patient with rheumatoid arthritis. Role for the Epstein-Barr virus. Rev Rhum Engl Ed 65:283–286
Niitsu N, Okamoto M, Nakamine H, Hirano M (2010) Clinicopathologic correlations of diffuse large B-cell lymphoma in rheumatoid arthritis patients treated with methotrexate. Cancer Sci 101:1309–1313
Shaffer DR, Rooney CM, Gottschalk S (2010) Immunotherapeutic options for Epstein-Barr virus-associated lymphoproliferative disease following transplantation. Immunotherapy 2:663–671
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kondo, S., Tanimoto, K., Yamada, K. et al. Mature T/NK-cell lymphoproliferative disease and Epstein–Barr virus infection are more frequent in patients with rheumatoid arthritis treated with methotrexate. Virchows Arch 462, 399–407 (2013). https://doi.org/10.1007/s00428-013-1389-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-013-1389-1