Abstract
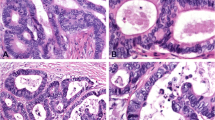
The most widely used system to define the histological grade of colorectal carcinoma (CRC) is based on the degree of gland formation. This system suffers from significant interobserver variability which may limit its prognostic value and consequently better standardized criteria for the assessment of histological grading of CRC are needed. The present study aims to evaluate and to compare, in a cohort of postsurgical pTNM stage I CRC, conventional histological grading, and a novel grading system based on the number of poorly differentiated clusters of neoplastic cells, in terms of interobserver reproducibility, prognostic significance on progression-free survival, and association with other clinicopathological characteristics. Grading with both systems was performed by two pathologists independently and blinded to the clinicopathological data. Interobserver agreement was higher when grade was assessed by counting poorly differentiated clusters than by assessing the relative proportion of the glandular component. Contrary to conventional grading, the novel system provided significant prognostic information in terms of progression-free survival and was significantly associated with budding, invasive growth, lymphatic invasion, and occult nodal metastases of CRC. In conclusion, our findings suggest that a tumor grading system based on the number of poorly differentiated clusters is more reproducible and provides better prognostic stratification of pTNM stage I CRC patients than conventional grading.



Similar content being viewed by others
References
Landis SH, Murray T, Bolden S (1999) Cancer statistics. Cancer J Clin 49:8–31
Wiggers T, Arends JW, Schutter B et al (1988) A multivariate analysis of pathologic prognostic indicators in large bowel cancer. Cancer 61:386–395
Wu XC, Chen VW, Steele B et al (2001) Subsite-specific incidence rate and stage of disease in colorectal cancer by race, gender, and age group. Cancer 92:2547–2554
Di Gregorio C, Fante R, Roncucci L et al (1996) Clinical features, frequency and prognosis of Dukes’ A colorectal carcinoma: a population-based investigation. Eur J Cancer 32A:1957–1962
Barresi V, Reggiani-Bonetti L, Di Gregorio C et al (2010) Stage I colorectal carcinoma: vascular endothelial growth factor (VEGF) immunohistochemical expression, microvessel density and their correlation with clinical outcome. Virchows Arch 457:11–19
Barresi V, Ieni A, Reggiani-Bonetti L, Di Gregorio C et al (2011) Neutrophil gelatinase-associated lipocalin (NGAL): a new prognostic marker in stage I colorectal carcinoma? Hum Pathol 42:1720–1726
Barresi V, Reggiani-Bonetti L, Di Gregorio C et al (2011) Lymphatic vessel density and its prognostic value in stage I colorectal carcinoma. J Clin Pathol 64:6–12
Barresi V, Reggiani-Bonetti L, Di Gregorio C et al (2011) Neutrophil gelatinase-associated lipocalin (NGAL) and matrix metalloproteinase-9 (MMP-9) prognostic value in stage I colorectal carcinoma. Pathol Res Pract 207:479–486
Barresi V, Reggiani-Bonetti L, Vitarelli E et al (2012) Immunohistochemical assessment of lymphovascular invasion in stage I colorectal carcinoma: prognostic relevance and correlation with nodal micrometastases. Am J Surg Pathol 36:66–72
Freedman LS, Macaskill P, Smith AN (1984) Multivariate analysis of prognostic factors for operable rectal cancer. Lancet 29:733–736
Fisher ER, Sass R, Palekar A et al (1989) Dukes’ classification revisited. Findings from the National Surgical Adjuvant Breast and Bowel Projects (Protocol R-01). Cancer 64:2354–2360
Blenkinsopp WK, Stewart-Brown S, Blesovsky L et al (1981) Histopathology reporting in large bowel cancer. J Clin Pathol 34:509–513
Thomas GDH, Dixon MF, Smeeton NC et al (1983) Observer variation in the histological grading of rectal carcinoma. J Clin Pathol 36:385–391
Chandler I, Houlston RS (2008) Interobserver agreement in grading of colorectal cancers-findings from a nationwide web-based survey of histopathologists. Histopathology 52:494–499
Ueno H, Kajiwara Y, Shimazaki H et al (2012) New criteria for histologic grading of colorectal cancer. Am J Surg Pathol 36:193–201
Hamilton SR, Volgelstein B, Kudo S et al (2000) Carcinoma of the colon and rectum. In: Hamilton SR, Aaltonen LA (eds) Pathology and genetics of tumours of the digestive system. IARC Press, Lyon, pp 110–111
Hamilton SR, Bosman FT, Boffetta P (2010) Carcinoma of the colon and rectum. In: Bosman T, Carneiro F, Hruban RH, Theise ND (eds) WHO classification of tumours of the digestive system. IARC Press, Lyon, pp 138–139
Sobin L, Gospodarowicz M, Wittekind C (2009) TNM classification of malignant tumours. Wiley, New York
Kikuchi R, Takano M, Takagi K et al (1995) Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum 38:1286–1895
Morodomi T, Isomoto H, Shirouzu G et al (1989) An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancers. Cancer 63:539–643
Ueno H, Mochizuki H, Hashiguchi Y et al (2004) Preoperative parameters expanding the indication of sphincter preserving surgery in patients with advanced low rectal cancer. Ann Surg 239:34–42
Reggiani Bonetti L, Di Gregorio C et al (2011) Lymph node micrometastasis and survival of patients with stage I (Dukes’ A) colorectal carcinoma. Scand J Gastroenterol 46:881–886
Lips DJ, Koebrugge B, Liefers GJ et al (2011) The influence of micrometastases on prognosis and survival in stage I–II colon cancer patients: the Enroute study. BMC Surg 11:11
Stewart FW, Spies JW (1929) Biopsy histology in the grading of rectal carcinoma. Am J Pathol 5:109–115
Dukes C (1937) Histological grading of rectal cancer. Proc R Soc Med 30:371–376
Grinnell RS (1939) The grading and prognosis of carcinoma of the colon and rectum. Ann Surg 109:500–533
Compton CC, Fielding LP, Burgart LJ et al (2000) Prognostic factors in colorectal cancer. College of American Pathologists consensus statement 1999. Arch Pathol Lab Med 124:979–994
Nicastri DG, Doucette JT, Godfrey TE et al (2007) Is occult lymph node disease in colorectal cancer patients clinically significant? A review of the relevant literature. J Mol Diagn 9:563–571
Greenson JK, Isenhart CE, Rice R et al (1994) Identification of occult micrometastases in pericolic lymph nodes of Duke’s B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer 73:563–569
Hayashi N, Ito I, Yanagisawa A, Kato Y et al (1995) Genetic diagnosis of lymph node metastasis in colorectal cancer. Lancet 345:1257–1259
Mescoli C, Albertoni L, Pucciarelli S et al (2012) Isolated tumor cells in regional lymph nodes as relapse predictors in stage I and II colorectal cancer. J Clin Oncol 30:965–971
Acknowledgments
We wish to thank Associazione Italiana Ricerca Cancro (AIRC) for financial support.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barresi, V., Reggiani Bonetti, L., Branca, G. et al. Colorectal carcinoma grading by quantifying poorly differentiated cell clusters is more reproducible and provides more robust prognostic information than conventional grading. Virchows Arch 461, 621–628 (2012). https://doi.org/10.1007/s00428-012-1326-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-012-1326-8