Abstract
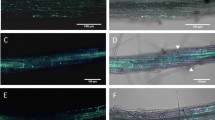
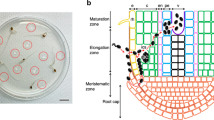
The compatible interaction between the model plant, Arabidopsis thaliana, and the GMI1000 strain of the phytopathogenic bacterium, Ralstonia solanacearum, was investigated in an in vitro pathosystem. We describe the progression of the bacteria in the root from penetration at the root surface to the xylem vessels and the cell type-specific, cell wall-associated modifications that accompanies bacterial colonization. Within 6 days post inoculation, R. solanacearum provoked a rapid plasmolysis of the epidermal, cortical, and endodermal cells, including those not directly in contact with the bacteria. Plasmolysis was accompanied by a global degradation of pectic homogalacturonanes as shown by the loss of JIM7 and JIM5 antibody signal in the cell wall of these cell types. As indicated by immunolabeling with Rsol-I antibodies that specifically recognize R. solanacearum, the bacteria progresses through the root in a highly directed, centripetal manner to the xylem poles, without extensive multiplication in the intercellular spaces along its path. Entry into the vascular cylinder was facilitated by cell collapse of the two pericycle cells located at the xylem poles. Once the bacteria reached the xylem vessels, they multiplied abundantly and moved from vessel to vessel by digesting the pit membrane between adjacent vessels. The degradation of the secondary walls of xylem vessels was not a prerequisite for vessel colonization as LM10 antibodies strongly labeled xylem cell walls, even at very late stages in disease development. Finally, the capacity of R. solanacearum to specifically degrade certain cell wall components and not others could be correlated with the arsenal of cell wall hydrolytic enzymes identified in the bacterial genome.








Similar content being viewed by others
Abbreviations
- dpi:
-
Days post-inoculation
- HG:
-
Homogalacturonan
- PEL:
-
Pectate lyase
- PG:
-
Polygalacturonase
- PL:
-
Pectin lyase
- PME:
-
Pectin methylesterase
- TEM:
-
Transmission electron microscopy
References
Alfano JR, Collmer A (2004) Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu Rev Phytopathol 42:385–414
Angot A, Peeters N, Lechner E, Vailleau F, Baud C, Gentzbittel L, Sartorel E, Genschik P, Boucher C, Genin S (2006) Ralstonia solanacearum requires F-box-like domain-containing type III effectors to promote disease on several host plants. Proc Natl Acad Sci USA 103:14620–14625
Arancon NQ, Edwards CA, Atiyeh R, Metzger JD (2004) Effects of vermicomposts produced from food waste on the growth and yields of greenhouse peppers. Bioresour Technol 93:139–144
Arlat M, Van Gijsegem F, Huet JC, Pernollet JC, Boucher CA (1994) PopA1, a protein which induces a hypersensitivity-like response on specific Petunia genotypes, is secreted via the Hrp pathway of Pseudomonas solanacearum. EMBO J 13:543–553
Aziz A, Heyraud A, Lambert B (2004) Oligogalacturonide signal transduction, induction of defense-related responses and protection of grapevine against Botrytis cinerea. Planta 218:767–774
Aziz A, Gauthier A, Bezier A, Poinssot B, Joubert JM, Pugin A, Heyraud A, Baillieul F (2007) Elicitor and resistance-inducing activities of beta-1,4 cellodextrins in grapevine, comparison with beta-1,3 glucans and alpha-1,4 oligogalacturonides. J Exp Bot 58:1463–1472
Blanvillain S, Meyer D, Boulanger A, Lautier M, Guynet C, Denancé N, Vasse J, Lauber E, Arlat M (2007) Plant carbohydrate scavenging through Tonb-dependent receptors: A feature shared by phytopathogenic and aquatic bacteria. PLoS One 2:e224
Boudjeko T, Andème-Onzighi C, Vicré M, Balangé A-P, Ndoumou DO, Driouich A (2006) Loss of pectin is an early event during infection of cocoyam roots by Pythium myriotylum. Planta 223:271–282
Burton RA, Gidley MJ, Fincher GB (2010) Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol 6:724–732
Campion C, Vian B, Nicole M, Rouxel F (1998) A comparative study of carrot root tissue colonization and cell wall degradation by Pythium violae and Pythium ultimum, two pathogens responsible for cavity spot. Can J Microbiol 44:221–230
Carpita NC (2011) Update on mechanisms of plant cell wall biosynthesis: how plants make cellulose and other (1 → 4)-{beta}-d-glycans. Plant Physiol 155:171–184
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Curvers K, Seifi H, Mouille G, de Rycke R, Asselbergh B, Van Hecke A, Vanderschaeghe D, Hofte H, Callewaert N, Van Breusegem F, Hofte M (2010) Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea. Plant Physiol 154:847–860
da Silva AC (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459–463
De Smet I, Vanneste S, Inze D, Beeckman T (2006) Lateral root initiation or the birth of a new meristem. Plant Mol Biol 60:871–887
Deslandes L, Pileur F, Liaubet L, Camut S, Can C, Williams K, Holub E, Beynon J, Arlat M, Marco Y (1998) Genetic characterization of RRS1, a recessive locus in Arabidopsis thaliana that confers resistance to the bacterial soilborne pathogen Ralstonia solanacearum. Mol Plant Microbe Interact 11:659–667
Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119:71–84
Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benkovà E (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Nat Acad Sci USA 105:8790–8794
Genin S (2010) Research review: molecular traits controlling host range and adaptation to plants in Ralstonia solanacearum. New Phytol 187:920–928
Gonzalez ET, Allen C (2003) Characterization of a Ralstonia solanacearum operon required for polygalacturonate degradation and uptake of galacturonic acid. Mol Plant Microbe Interact 16:536–544
Hayward AC (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas Solanacearum. Annu Rev Phytopathol 29:65–87
Hernandez-Blanco C, Feng DX, Hu J, Sanchez-Vallet A, Deslandes L, Llorente F, Berrocal-Lobo M, Keller H, Barlet X, Sanchez-Rodriguez C, Anderson LK, Somerville S, Marco Y, Molina A (2007) Impairment of cellulose synthases required for arabidopsis secondary cell wall formation enhances disease resistance. Plant Cell 19:890–903
Hu J, Barlet X, Deslandes L, Hirsch J, Feng DX, Somssich I, Marco Y (2008) Transcriptional responses of Arabidopsis thaliana during wilt disease caused by the soil-borne phytopathogenic bacterium, Ralstonia solanacearum. PLoS One 3:e2589
Huang Q, Allen C (1997) An exo-poly-alpha-d-galacturonosidase, PehB, is required for wild-type virulence of Ralstonia solanacearum. J Bacteriol 179:7369–7378
Huckelhoven R (2007) Cell wall-associated mechanisms of disease resistance and susceptibility. Annu Rev Phytopathol 45:101–127
Liu H, Zhang S, Schell MA, Denny TP (2005) Pyramiding unmarked deletions in Ralstonia solanacearum shows that secreted proteins in addition to plant cell-wall-degrading enzymes contribute to virulence. Mol Plant Microbe Interact 18:1296–1305
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioessays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakaho K, Hibino H, Miyagawa H (2000) Possible mechanisms limiting movement of Ralstonia solanacearum in resistant tomato tissues. J Phytopathol 148:181–190
Oeser B, Heidrich PM, Müller U, Tudzynski P, Tenberge KB (2002) Polygalacturonase is a pathogenicity factor in the Claviceps purpurea/rye interaction. Fungal Genet Biol 36:176–186
Osorio S, Castillejo C, Quesada MA, Medina-Escobar N, Brownsey GJ, Suau R, Heredia A, Botella MA, Valpuesta V (2008) Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca). Plant J 54:43–55
Parizot B, Laplaze L, Ricaud L, Boucheron-Dubuisson E, Bayle V, Bonke M, De Smet I, Poethig SR, Helariutta Y, Haseloff J, Chriqui D, Beeckman T, Nussaume L (2008) Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiol 146:140–148
Pattathil S, Avci U, Baldwin D, Swennes AG, McGill JA, Popper Z, Bootten T, Albert A, Davis RH, Chennareddy C, Dong R, O’Shea B, Rossi R, Leoff C, Freshour G, Narra R, O’Neil M, York WS, Hahn MG (2010) A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol 153:514–525
Plener L, Manfredi P, Valls M, Genin S (2010) PrhG, a transcriptional regulator responding to growth conditions, is involved in the control of the type III secretion system regulon in Ralstonia solanacearum. J Bacteriol 192:1011–1019
Poueymiro M, Genin S (2009) Secreted proteins from Ralstonia solanacearum: a hundred tricks to kill a plant. Curr Opin Microbiol 12:44–52
Saile E, McGarvey JA, Schell MA, Denny TP (1997) Role of extracellular polysaccharide and endoglucanase in root invasion and colonization of tomato plants by Ralstonia solanacearum. Phytopathology 87:1264–1271
Schacht T, Unger C, Pich A, Wydra K (2011) Endo- and exopolygalacturonases of Ralstonia solanacearum are inhibited by polygalacturonase-inhibiting protein (PGIP) activity in tomato stem extracts. Plant Physiol Biochem 49:377–387
Schell MA, Roberts DP, Denny TP (1988) Analysis of the Pseudomonas solanacearum polygalacturonase encoded by pglA and its involvement in phytopathogenicity. J Bacteriol 170:4501–4508
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61:263–289
Tans-Kersten J, Guan Y, Allen C (1998) Ralstonia solanacearum pectin methylesterase is required for growth on methylated pectin but not for bacterial wilt virulence. Appl Environ Microbiol 64:4918–4923
Tasset C, Bernoux M, Jauneau A, Pouzet C, Brière C, Kieffer-Jacquinod S, Rivas S, Marco Y, Deslandes L (2010) Autoacetylation of the Ralstonia solanacearum effector PopP2 targets a lysine residue essential for RRS1-R-mediated immunity in Arabidopsis. PLoS Pathog 6:e1001202
Thiery J (1967) Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J Microscopie 6:987–1018
Turner M, Jauneau A, Genin S, Tavella MJ, Vailleau F, Gentzbittel L, Jardinaud MF (2009) Dissection of bacterial wilt on Medicago truncatula revealed two type III secretion system effectors acting on root infection process and disease development. Plant Physiol 150:1713–1722
Vailleau F, Sartorel E, Jardinaud MF, Chardon F, Genin S, Huguet T, Gentzbittel L, Petitprez M (2007) Characterization of the interaction between the bacterial wilt pathogen Ralstonia solanacearum and the model legume plant Medicago truncatula. Mol Plant Microbe Interact 20:159–167
Valette-Collet O, As Cimerman, Reignault P, Levis C, Boccara M (2003) Disruption of Botrytis cinerea pectin methylesterase gene Bcpme1 reduces virulence on several host plants. Mol Plant Microbe Interact 16:360–367
Vallenet D, Labarre L, Rouy Z, Barbe V, Bocs S, Cruveiller S, Lajus A, Pascal G, Scarpelli C, Médigue C (2006) MaGe: a microbial genome annotation system supported by synteny results. Nucleic Acids Res 34:53–65
Vasse J, Frey P, Trigalet A (1995) Microscopic studies of intercellular infection and protoxylem invasion of tomato roots by Pseudomonas solanacearum. Mol Plant Microbe Interact 8:241–251
Vasse J, Genin S, Frey P, Boucher C, Brito B (2000) The hrpB and hrpG regulatory genes of Ralstonia solanacearum are required for different stages of the tomato root infection process. Mol Plant Microbe Interact 13:259–267
Vogel J, Somerville S (2000) Isolation and characterization of powdery mildew-resistant Arabidopsis mutants. Proc Natl Acad Sci USA 97:1897–1902
Vogel JP, Raab TK, Schiff C, Somerville SC (2002) PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell 14:2095–2106
Vogel JP, Raab TK, Somerville CR, Somerville SC (2004) Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J 40:968–978
Wydra K, Beri H (2006) Structural changes of homogalacturonan, rhamnogalacturonan I and arabinogalactan protein in xylem cell walls of tomato genotypes in reaction to Ralstonia solanacearum. Physiol Mol Plant Pathol 68:41–50
Wydra K, Beri H (2007) Immunohistochemical changes in methyl-ester distribution of homogalacturonan and side chain composition of rhamnogalacturonan I as possible components of basal resistance in tomato inoculated with Ralstonia solanacearum. Physiol Mol Plant Pathol 70:13–24
Zhu Y, Nam J, Carpita NC, Matthysse AG, Gelvin SB (2003a) Agrobacterium-mediated root transformation is inhibited by mutation of an Arabidopsis cellulose synthase-like gene. Plant Physiol 133:1000–1010
Zhu Y, Nam J, Humara JM, Mysore KS, Lee LY, Cao H, Valentine L, Li J, Kaiser AD, Kopecky AL, Hwang HH, Bhattacharjee S, Rao PK, Tzfira T, Rajagopal J, Yi H, Veena, Yadav BS, Crane YM, Lin K, Larcher Y, Gelvin MJ, Knue M, Ramos C, Zhao X, Davis SJ, Kim SI, Ranjith-Kumar CT, Choi YJ, Hallan VK, Chattopadhyay S, Sui X, Ziemienowicz A, Matthysse AG, Citovsky V, Hohn B, Gelvin SB (2003b) Identification of Arabidopsis rat mutants. Plant Physiol 132:494–505
Zolobowska L, Van Gijsegem F (2006) Induction of lateral root structure formation on petunia roots: a novel effect of GMI1000 Ralstonia solanacearum infection impaired in Hrp mutants. Mol Plant Microbe Interact 19:597–606
Acknowledgments
The authors would like to thank Stéphane Genin and Alice Guidot (LIPM, Toulouse, France) for helpful discussions, and Françoise Poliakoff, Corrine Audusseau and Carène Rivoal (ANSES-Laboratoire de la Santé des Végétaux, Angers, France) for the generous gift of Rsol-I antibody. This work was funded by the French National Agency for Research (WALLTALK: ANR-07-GPLA-014).
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Digonnet and Y. Martinez contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Digonnet, C., Martinez, Y., Denancé, N. et al. Deciphering the route of Ralstonia solanacearum colonization in Arabidopsis thaliana roots during a compatible interaction: focus at the plant cell wall. Planta 236, 1419–1431 (2012). https://doi.org/10.1007/s00425-012-1694-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-012-1694-y