Abstract
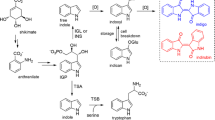
A type III polyketide synthase cDNA and the corresponding gene (PcPKS2) were cloned from Polygonum cuspidatum Sieb. et Zucc. Sequencing results showed that the ORF of PcPKS2 was interrupted by three introns, which was an unexpected finding because all type III PKS genes studied so far contained only one intron at a conserved site in flowering plants, except for an Antirrhinum majus chalcone synthase gene. Besides the unusual gene structure, PcPKS2 showed some interesting characteristics: (1) the CHS “gatekeepers” Phe215 and Phe265 are uniquely replaced by Leu and Cys, respectively; (2) recombinant PcPKS2 overexpressed in Escherichia coli efficiently afforded 4-coumaroyltriacetic acid lactone (CTAL) as a major product along with bis-noryangonin (BNY) and p-hydroxybenzalacetone at low pH; however, it effectively yielded p-hydroxybenzalacetone as a dominant product along with CTAL and BNY at high pH. Beside p-hydroxybenzalacetone, CTAL and BNY, a trace amount of naringenin chalcone could be detected in assays at different pH. Furthermore, 4-coumaroyl-CoA and feruloyl-CoA were the only cinnamoyl-CoA derivatives accepted as starter substrates. PcPKS2 did not accept isobutyryl-CoA, isovaleryl-CoA or acetyl-CoA as substrate. DNA gel blot analysis indicated that there are two to four PcPKS2 copies in the P. cuspidatum genome. RNA gel blot analysis revealed that PcPKS2 is highly expressed in the rhizomes and in young leaves, but not in the roots of the plant. PcPKS2 transcripts in leaves were induced by pathogen infection, but not by wounding.








Similar content being viewed by others
Abbreviations
- ALS:
-
Aloesone synthase
- BAS:
-
Benzalacetone synthase
- CHS:
-
Chalcone synthase
- CoA:
-
Coenzyme A
- CTAS:
-
p-Coumaroyl triacetic acid synthase
- LC-ESIMS:
-
Liquid chromatography electron spray ionization mass spectrometry
- OKS:
-
Octaketide synthase
- PKS:
-
Polyketide synthase
- STS:
-
Stilbene synthase
References
Abe I, Takahashi Y, Morita H, Noguchi H (2001) Benzalacetone synthase. A novel polyketide synthase that plays a crucial role in the biosynthesis of phenylbutanones in Rheum palmatum. Eur J Biochem 268:3354–3359
Abe I, Takahashi Y, Noguchi H (2002) Enzymatic formation of an unnatural C(6)-C(5) aromatic polyketide by plant type III polyketide synthases. Org Lett 4:3623–3626
Abe I, Sano Y, Takahashi Y, Noguchi H (2003) Site-directed mutagenesis of benzalacetone synthase. The role of the Phe215 in plant type III polyketide synthases. J Biol Chem 278:25218–25226
Abe I, Utsumi Y, Oguro S, Noguchi H (2004) The first plant type III polyketide synthase that catalyzes formation of aromatic heptaketide. FEBS Lett 562:171–176
Abe I, Oguro S, Utsumi Y, Sano Y, Noguchi H (2005) Engineered biosynthesis of plant polyketides: chain length control in an octaketide-producing plant type III polyketide synthase. J Am Chem Soc 127:12709–12716
Abe T, Morita H, Noma H, Kohno T, Noguchi H, Abe I (2007) Structure function analysis of benzalacetone synthase from Rheum palmatum. Bioorg Med Chem 17:3161–3166
Akiyama T, Shibuya M, Liu HM, Ebizuka Y (1999) p-Coumaroyltriacetic acid synthase, a new homologue of chalcone synthase, from Hydrangea macrophylla var. thunbergii. Eur J Biochem 263:834–839
Austin MB, Noel JP (2003) The chalcone synthase superfamily of type III polyketide synthases. Nat Prod Rep 20:79–110
Beekwilder J, van der Meer IM, Sibbesen O, Broekgaarden M, Qvist I, Mikkelsen JD, Hall RD (2007) Microbial production of natural raspberry ketone. Biotechnol J 2:1270–1279
Beuerle T, Pichersky E (2002) Enzymatic synthesis and purification of aromatic coenzyme a esters. Anal Biochem 302:305–312
Blacklock BJ, Jaworski JG (2006) Substrate specificity of Arabidopsis 3-ketoacyl-CoA synthases. Biochem Biophys Res Comm 346:583–590
Borejsza-Wysocki W, Hrazdina G (1994) Biosynthesis of p-hydroxyphenylbutan-2-one in raspberry fruits and tissue culture. Phytochemistry 35:623–628
Borejsza-Wysocki W, Hrazdina G (1996) Aromatic polyketide synthases (purification, characterization, and antibody development to benzalacetone synthase from raspberry fruits). Plant Physiol 110:791–799
Buchanan BB, Gruissem W, Jones RL (2002) Biochemistry & molecular biology of plants. American Society of Plant Physiologists, Waldorf
Caldwell MM, Robberecht R, Fint SD (1983) Internal filters: prospects for UV-acclimation in higher plants. Physiol Plant 58:445–450
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Durbin ML, McCaig B, Clegg MT (2000) Molecular evolution of the chalcone synthase multigene family in the morning glory genome. Plant Mol Biol 42:79–92
Ferrer JL, Jez JM, Bowman ME, Dixon RA, Noel JP (1999) Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat Struct Biol 6:775–784
Hegde VR, Pu H, Patel M, Black T, Soriano A, Zhao W, Gullo VP, Chan TM (2004) Two new bacterial DNA primase inhibitors from the plant Polygonum cuspidatum. Bioorg Med Chem 14:2275–2277
Hrazdina G, Kreuzaler F, Hahlbrock K, Grisebach H (1976) Substrate specificity of flavanone synthase from cell suspension cultures of parsley and structure of release products in vitro. Arch Biochem Biophys 175:392–399
James DW Jr, Lim E, Keller J, Plooy I, Ralston E, Dooner HK (1995) Directed tagging of the Arabidopsis FATTY ACID ELONGATION1 (FAE1) gene with the maize transposon activator. Plant Cell 7:309–319
Jiang C, Schommer CK, Kim SY, Suh DY (2006) Cloning and characterization of chalcone synthase from the moss, Physcomitrella patens. Phytochemistry 67:2531–2540
Kajikawa M, Yamaoka S, Yamato KT, Kanamaru H, Sakuradani E, Shimizu S, Fukuzawa H, Ohyama K (2003a) Functional analysis of a beta-ketoacyl-CoA synthase gene, MpFAE2, by gene silencing in the liverwort Marchantia polymorpha L. Biosci Biotech Bioch 67:605–612
Kajikawa M, Yamato KT, Kanamaru H, Sakuradani E, Shimizu S, Fukuzawa H, Sakai Y, Ohyama K (2003b) MpFAE3, a beta-ketoacyl-CoA synthase gene in the liverwort Marchantia polymorpha L., is preferentially involved in elongation of palmitic acid to stearic acid. Biosci Biotech Bioch 67:1667–1674
Kauppinen S (1992) Structure and expression of the Kas12 gene encoding a beta-ketoacyl-acyl carrier protein synthase I isozyme from barley. J Biol Chem 267:23999–24006
Liu B, Falkenstein-Paul H, Schmidt W, Beerhues L (2003) Benzophenone synthase and chalcone synthase from Hypericum androsaemum cell cultures: cDNA cloning, functional expression, and site-directed mutagenesis of two polyketide synthases. Plant J 34:847–855
Lorkovic ZJ, Wieczorek Kirk DA, Lambermon MH, Filipowicz W (2000) Pre-mRNA splicing in higher plants. Trends Plant Sci 5:160–167
Mallet J, Houhou L, Pajak F, Oda Y, Cervini R, Bejanin S, Berrard S (1998) The cholinergic locus: ChAT and VAChT genes. J Physiol Paris 92:145–147
Matsuda H, Shimoda H, Uemura T, Ueda T, Yamahara J, Yoshikawa M (1999) Chemical constituents from the leaves of Hydrangea macrophylla var. thunbergii (III): Absolute stereostructures of hydramacrosides A and B, secoiridoid glucoside complexes with inhibitory activity on histamine release. Chem Pharm Bull 47:1753–1758
Morita H, Takahashi Y, Noguchi H, Abe I (2000) Enzymatic formation of unnatural aromatic polyketides by chalcone synthase. Biochem Biophys Res Comm 279:190–195
Nonomura S, Kanagawa H, Makimoto A (1963) Chemical constituents of polygonaceous plants. I. Studies on the components of Ko-J O-Kon. (Polygonum Cuspidatum Sieb. Et Zucc.). Yakugaku Zasshi 83:988–990
Page RD (1996) Tree view: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Porebski S, Bailey L, Baum B (1997) Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep 15:8–15
Reddy AS (2007) Alternative splicing of pre-messenger RNAs in plants in the genomic era. Annu Rev Plant Biol 58:267–294
Reimold U, Kroger M, Kreuzaler F, Hahlbrock K (1983) Coding and 3’ non-coding nucleotide sequence of chalcone synthase mRNA and assignment of amino acid sequence of the enzyme. EMBO J 2:1801–1805
Samappito S, Page JE, Schmidt J, De-Eknamkul W, Kutchan TM (2003) Aromatic and pyrone polyketides synthesized by a stilbene synthase from Rheum tataricum. Phytochemistry 62:313–323
Sambrooke J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Schröder J (1997) A family of plant-specific polyketide synthases: facts and predictions. Trends Plant Sci 2:373–378
Sommer H, Saedler H (1986) Structure of the chalcone synthase gene of Antirrhinum majus. Mol Gen Genet 202:429–434
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Todd J, Post-Beitenmiller D, Jaworski JG (1999) KCS1 encodes a fatty acid elongase 3-ketoacyl-CoA synthase affecting wax biosynthesis in Arabidopsis thaliana. Plant J 17:119–130
Tosi M (1998) Molecular genetics of C1 inhibitor. Immunobiology 199:358–365
Trapp SC, Croteau RB (2001) Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 158:811–832
Verma D (1992) Signals in root nodule organogenesis and endocytosis of Rhizobium. Plant Cell 4:373–382
Xiao K, Xuan L, Xu Y, Bai D, Zhong D (2002) Constituents from Polygonum cuspidatum. Chem Pharm Bull 50:605–608
Yi T, Zhang H, Cai Z (2007) Analysis of rhizoma Polygoni Cuspidati by HPLC and HPLC-ESI/MS. Phytochem Anal 18:387–392
Yoshikawa M, Ueda T, Matsuda H, Yamahara J, Murakami N (1994) Absolute stereostructures of hydramacrosides A and B, new bioactive secoiridoid glucoside complexes from the leaves of Hydrangea macrophylla Seringe var. thunbergii Makino. Chem Pharm Bull 42:1691–1693
Zheng D, Hrazdina G (2008) Molecular and biochemical characterization of benzalacetone synthase and chalcone synthase genes and their proteins from raspberry (Rubus idaeus L.). Arch Biochem Biophys 470:139–145
Zheng D, Schroder G, Schroder J, Hrazdina G (2001) Molecular and biochemical characterization of three aromatic polyketide synthase genes from Rubus idaeus. Plant Mol Biol 46:1–15
Acknowledgments
The authors wish to thank Ms. Hong Yan, State Key Laboratory of Plant Physiology and Biochemistry, China Agricultural University, for help with the HPLC experiments.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ma, LQ., Pang, XB., Shen, HY. et al. A novel type III polyketide synthase encoded by a three-intron gene from Polygonum cuspidatum . Planta 229, 457–469 (2009). https://doi.org/10.1007/s00425-008-0845-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0845-7