Abstract
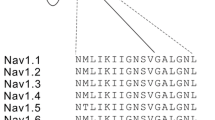
Mutations in voltage-gated sodium channels are associated with altered pain perception in humans. Most of these mutations studied to date present with a direct and intuitive link between the altered electrophysiological function of the channel and the phenotype of the patient. In this study, we characterize a variant of Nav1.8, D1639N, which has been previously identified in a patient suffering from the chronic pain syndrome “small fiber neuropathy”. Using a heterologous expression system and patch-clamp analysis, we show that Nav1.8/D1639N reduces current density without altering biophysical gating properties of Nav1.8. Therefore, the D1639N variant causes a loss-of-function of the Nav1.8 sodium channel in a patient suffering from chronic pain. Using immunocytochemistry and biochemical approaches, we show that Nav1.8/D1639N impairs trafficking of the channel to the cell membrane. Neither co-expression of β1 or β3 subunit, nor overnight incubation at 27 °C rescued current density of the D1639N variant. On the other hand, overnight incubation with lidocaine fully restored current density of Nav1.8/D1639N most likely by overcoming the trafficking defect, whereas phenytoin failed to do so. Since lidocaine rescues the loss-of-function of Nav1.8/D1639N, it may offer a future therapeutic option for the patient carrying this variant. These results demonstrate that the D1639N variant, identified in a patient suffering from chronic pain, causes loss-of-function of the channel due to impaired cell surface trafficking and that this trafficking defect can be rescued by lidocaine.





Similar content being viewed by others
References
Akopian AN, Sivilotti L, Wood JN (1996) A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379:257–262. https://doi.org/10.1038/379257a0
Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN (1999) The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci 2:541–548. https://doi.org/10.1038/9195
Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ, January CT (2006) Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113:365–373. https://doi.org/10.1161/CIRCULATIONAHA.105.570200
Becker D, Woltersdorf R, Boldt W, Schmitz S, Braam U, Schmalzing G, Markwardt F (2008) The P2X7 carboxyl tail is a regulatory module of P2X7 receptor channel activity. J Biol Chem 283:25725–25734. https://doi.org/10.1074/jbc.M803855200
Bökel C, Dass S, Wilsch-Bräuninger M, Roth S (2006) Drosophila Cornichon acts as cargo receptor for ER export of the TGFα-like growth factor Gurken. Development 133:459–470. https://doi.org/10.1242/dev.02219
Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD (1992) Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res 592:283–297
Castro CP, Piscopo D, Nakagawa T, Derynck R (2007) Cornichon regulates transport and secretion of TGFα-related proteins in metazoan cells. J Cell Sci 120:2454–2466. https://doi.org/10.1242/jcs.004200
Chahine M, O’Leary ME (2011) Regulatory role of voltage-gated Na+ channel β subunits in sensory neurons. Front Pharmacol 2:70. https://doi.org/10.3389/fphar.2011.00070
Chevrier P, Vijayaragavan K, Chahine M (2004) Differential modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by the local anesthetic lidocaine. Br J Pharmacol 142:576–584. https://doi.org/10.1038/sj.bjp.0705796
Cummins TR (1997) Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci 17:3503–3514
Dabby R, Sadeh M, Broitman Y, Yosovich K, Dickman R, Leshinsky-Silver E (2016) Painful small fiber neuropathy with gastroparesis: a new phenotype with a novel mutation in the SCN10A gene. J Clin Neurosci 26:84–88. https://doi.org/10.1016/j.jocn.2015.05.071
Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ (1992) Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 358:761–764. https://doi.org/10.1038/358761a0
Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN (2003) The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol 550:739–752. https://doi.org/10.1113/jphysiol.2003.042127
Faber CG, Lauria G, Merkies ISJ, Cheng X, Han C, Ahn H-S, Persson A-K, Hoeijmakers JGJ, Gerrits MM, Pierro T, Lombardi R, Kapetis D, Dib-Hajj SD, Waxman SG (2012) Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci U S A 109:19444–19449. https://doi.org/10.1073/pnas.1216080109
Fallah G, Römer T, Detro-Dassen S, Braam U, Markwardt F, Schmalzing G (2011) TMEM16A(a)/anoctamin-1 shares a homodimeric architecture with CLC chloride channels. Mol Cell Proteomics MCP 10:M110.004697. https://doi.org/10.1074/mcp.M110.004697
Gautron L, Sakata I, Udit S, Zigman JM, Wood JN, Elmquist JK (2011) Genetic tracing of Nav1.8-expressing vagal afferents in the mouse. J Comp Neurol 519:3085–3101. https://doi.org/10.1002/cne.22667
Gibson R, Schlesinger S, Kornfeld S (1979) The nonglycosylated glycoprotein of vesicular stomatitis virus is temperature-sensitive and undergoes intracellular aggregation at elevated temperatures. J Biol Chem 254:3600–3607
Gloor S, Pongs O, Schmalzing G (1995) A vector for the synthesis of cRNAs encoding Myc epitope-tagged proteins in Xenopus laevis oocytes. Gene 160:213–217
Haeger S, Kuzmin D, Detro-Dassen S, Lang N, Kilb M, Tsetlin V, Betz H, Laube B, Schmalzing G (2010) An intramembrane aromatic network determines pentameric assembly of Cys-loop receptors. Nat Struct Mol Biol 17:90–98. https://doi.org/10.1038/nsmb.1721
Han C, Vasylyev D, Macala LJ, Gerrits MM, Hoeijmakers JGJ, Bekelaar KJ, Dib-Hajj SD, Faber CG, Merkies ISJ, Waxman SG (2014) The G1662S NaV1.8 mutation in small fibre neuropathy: impaired inactivation underlying DRG neuron hyperexcitability. J Neurol Neurosurg Psychiatry 85:499–505. https://doi.org/10.1136/jnnp-2013-306095
Hoeijmakers JG, Faber CG, Lauria G, Merkies IS, Waxman SG (2012) Small-fibre neuropathies—advances in diagnosis, pathophysiology and management. Nat Rev Neurol 8:369–379. https://doi.org/10.1038/nrneurol.2012.97
Hoshino H, Uchida T, Otsuki T, Kawamoto S, Okubo K, Takeichi M, Chisaka O (2007) Cornichon-like protein facilitates secretion of HB-EGF and regulates proper development of cranial nerves. Mol Biol Cell 18:1143–1152. https://doi.org/10.1091/mbc.E06-08-0733
Hu D, Barajas-Martínez H, Pfeiffer R, Dezi F, Pfeiffer J, Buch T, Betzenhauser MJ, Belardinelli L, Kahlig KM, Rajamani S, DeAntonio HJ, Myerburg RJ, Ito H, Deshmukh P, Marieb M, Nam G-B, Bhatia A, Hasdemir C, Haïssaguerre M, Veltmann C, Schimpf R, Borggrefe M, Viskin S, Antzelevitch C (2014) Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J Am Coll Cardiol 64:66–79. https://doi.org/10.1016/j.jacc.2014.04.032
Huang J, Yang Y, Zhao P, Gerrits MM, Hoeijmakers JGJ, Bekelaar K, Merkies ISJ, Faber CG, Dib-Hajj SD, Waxman SG (2013) Small-fiber neuropathy Nav1.8 mutation shifts activation to hyperpolarized potentials and increases excitability of dorsal root ganglion neurons. J Neurosci 33:14087–14097. https://doi.org/10.1523/JNEUROSCI.2710-13.2013
Huang J, Vanoye CG, Cutts A, Goldberg YP, Dib-Hajj SD, Cohen CJ, Waxman SG, George AL (2017) Sodium channel NaV1.9 mutations associated with insensitivity to pain dampen neuronal excitability. J Clin Invest 127:2805–2814. https://doi.org/10.1172/JCI92373
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110:1389–1398. https://doi.org/10.1172/JCI16886
Kirsch R, Joly E (1998) An improved PCR-mutagenesis strategy for two-site mutagenesis or sequence swapping between related genes. Nucleic Acids Res 26:1848–1850. https://doi.org/10.1093/nar/26.7.1848
Kist AM, Sagafos D, Rush AM, Neacsu C, Eberhardt E, Schmidt R, Lunden LK, Ørstavik K, Kaluza L, Meents J, Zhang Z, Carr TH, Salter H, Malinowsky D, Wollberg P, Krupp J, Kleggetveit IP, Schmelz M, Jørum E, Lampert A, Namer B (2016) SCN10A mutation in a patient with erythromelalgia enhances C-fiber activity dependent slowing. PLoS One 11:e0161789. https://doi.org/10.1371/journal.pone.0161789
Leipold E, Liebmann L, Korenke GC, Heinrich T, Gießelmann S, Baets J, Ebbinghaus M, Goral RO, Stödberg T, Hennings JC, Bergmann M, Altmüller J, Thiele H, Wetzel A, Nürnberg P, Timmerman V, De Jonghe P, Blum R, Schaible H-G, Weis J, Heinemann SH, Hübner CA, Kurth I (2013) A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat Genet 45:1399–1404. https://doi.org/10.1038/ng.2767
Li Q, Su Y-Y, Wang H, Li L, Wang Q, Bao L (2010) Transmembrane segments prevent surface expression of sodium channel Nav1.8 and promote calnexin-dependent channel degradation. J Biol Chem 285:32977–32987. https://doi.org/10.1074/jbc.M110.143024
Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC (1998) Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci 18:2174–2187
Núñez L, Barana A, Amorós I, de la Fuente MG, Dolz-Gaitón P, Gómez R, Rodríguez-García I, Mosquera I, Monserrat L, Delpón E, Caballero R, Castro-Beiras A, Tamargo J (2013) p.D1690N Nav1.5 rescues p.G1748D mutation gating defects in a compound heterozygous Brugada syndrome patient. Heart Rhythm 10:264–272. https://doi.org/10.1016/j.hrthm.2012.10.025
Okuse K, Malik-Hall M, Baker MD, Poon W-YL, Kong H, Chao MV, Wood JN (2002) Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature 417:653–656. https://doi.org/10.1038/nature00781
Perez K, Yeam I, Jahn MM, Kang B-C (2006) Megaprimer-mediated domain swapping for construction of chimeric viruses. J Virol Methods 135:254–262. https://doi.org/10.1016/j.jviromet.2006.03.020
Ragsdale DS, McPhee JC, Scheuer T, Catterall WA (1996) Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A 93:9270–9275
Ramachandra R, McGrew SY, Baxter JC, Kiveric E, Elmslie KS (2012) Tetrodotoxin-resistant voltage-dependent sodium channels in identified muscle afferent neurons. J Neurophysiol 108:2230–2241. https://doi.org/10.1152/jn.00219.2012
Ramachandra R, McGrew SY, Baxter JC, Howard JR, Elmslie KS (2013) NaV1.8 channels are expressed in large, as well as small, diameter sensory afferent neurons. Channels Austin Tex 7:34–37. https://doi.org/10.4161/chan.22445
Renganathan M, Cummins TR, Waxman SG (2001) Contribution of Nav1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol 86:629–640
Ronstedt K, Sternberg D, Detro-Dassen S, Gramkow T, Begemann B, Becher T, Kilian P, Grieschat M, Machtens J-P, Schmalzing G, Fischer M, Fahlke C (2015) Impaired surface membrane insertion of homo- and heterodimeric human muscle chloride channels carrying amino-terminal myotonia-causing mutations. Sci Rep 5:15382. https://doi.org/10.1038/srep15382
Rusconi R, Scalmani P, Cassulini RR, Giunti G, Gambardella A, Franceschetti S, Annesi G, Wanke E, Mantegazza M (2007) Modulatory proteins can rescue a trafficking defective epileptogenic Nav1.1 Na+ channel mutant. J Neurosci 27:11037–11046. https://doi.org/10.1523/JNEUROSCI.3515-07.2007
Rusconi R, Combi R, Cestèle S, Grioni D, Franceschetti S, Dalprà L, Mantegazza M (2009) A rescuable folding defective Nav1.1 (SCN1A) sodium channel mutant causes GEFS+: common mechanism in Nav1.1 related epilepsies? Hum Mutat 30:E747–E760. https://doi.org/10.1002/humu.21041
Schwarz DS, Blower MD (2016) The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci 73:79–94. https://doi.org/10.1007/s00018-015-2052-6
Schwenk J, Harmel N, Zolles G, Bildl W, Kulik A, Heimrich B, Chisaka O, Jonas P, Schulte U, Fakler B, Klöcker N (2009) Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 323:1313–1319. https://doi.org/10.1126/science.1167852
Shields SD, Ahn H, Yang Y, Han C, Seal RP, Wood JN, Waxman SG, Dib-hajj SD (2012) Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain 153:2017–2030. https://doi.org/10.1016/j.pain.2012.04.022
Stolz M, Klapperstück M, Kendzierski T, Detro-Dassen S, Panning A, Schmalzing G, Markwardt F (2015) Homodimeric anoctamin-1, but not homodimeric anoctamin-6, is activated by calcium increases mediated by the P2Y1 and P2X7 receptors. Pflugers Arch 467:2121–2140. https://doi.org/10.1007/s00424-015-1687-3
Swanwick RS, Pristerá A, Okuse K (2010) The trafficking of NaV1.8. Neurosci Lett 486:78–83. https://doi.org/10.1016/j.neulet.2010.08.074
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13:184–190. https://doi.org/10.1038/ncb0311-184
Terragni B, Scalmani P, Franceschetti S, Cestèle S, Mantegazza M (2017) Post-translational dysfunctions in channelopathies of the nervous system. Neuropharmacology 132:31–42. https://doi.org/10.1016/j.neuropharm.2017.05.028
Thomas D, Kiehn J, Katus HA, Karle CA (2003) Defective protein trafficking in hERG-associated hereditary long QT syndrome (LQT2): molecular mechanisms and restoration of intracellular protein processing. Cardiovasc Res 60:235–241. https://doi.org/10.1016/j.cardiores.2003.08.002
Valdivia CR, Tester DJ, Rok BA, Porter C-BJ, Munger TM, Jahangir A, Makielski JC, Ackerman MJ (2004) A trafficking defective, Brugada syndrome-causing SCN5A mutation rescued by drugs. Cardiovasc Res 62:53–62. https://doi.org/10.1016/j.cardiores.2004.01.022
van den Boogaard M, Smemo S, Burnicka-Turek O, Arnolds DE, van de Werken HJG, Klous P, McKean D, Muehlschlegel JD, Moosmann J, Toka O, Yang XH, Koopmann TT, Adriaens ME, Bezzina CR, de Laat W, Seidman C, Seidman JG, Christoffels VM, Nobrega MA, Barnett P, Moskowitz IP (2014) A common genetic variant within SCN10A modulates cardiac SCN5A expression. J Clin Invest 124:1844–1852. https://doi.org/10.1172/JCI73140
Vijayaragavan K, Powell AJ, Kinghorn IJ, Chahine M (2004) Role of auxiliary beta1-, beta2-, and beta3-subunits and their interaction with Na(v)1.8 voltage-gated sodium channel. Biochem Biophys Res Commun 319:531–540. https://doi.org/10.1016/j.bbrc.2004.05.026
Waxman SG, Kocsis JD, Black JA (1994) Type III sodium channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is reexpressed following axotomy. J Neurophysiol 72:466–470
Weiner MP, Costa GL, Schoettlin W, Cline J, Mathur E, Bauer JC (1994) Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene 151:119–123
Wong H-K, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, Strooper BD, Saftig P, Nukina N (2005) β subunits of voltage-gated sodium channels are novel substrates of β-site amyloid precursor protein-cleaving enzyme (BACE1) and γ-secretase. J Biol Chem 280:23009–23017. https://doi.org/10.1074/jbc.M414648200
Zeng Z, Zeng Z, Xie Q, Xie Q, Huang Y, Huang Y, Zhao Y, Zhao Y, Li W, Li W, Huang Z, Huang Z (2016) p.D1690N sodium voltage-gated channel α subunit 5 mutation reduced sodium current density and is associated with Brugada syndrome. Mol Med Rep 13:5216–5222
Zhao J, Ziane R, Chatelier A, O’Leary ME, Chahine M (2007) Lidocaine promotes the trafficking and functional expression of Nav1.8 sodium channels in mammalian cells. J Neurophysiol 98:467–477. https://doi.org/10.1152/jn.00117.2007
Zhao J, O’Leary ME, Chahine M (2011) Regulation of Nav1.6 and Nav1.8 peripheral nerve Na+ channels by auxiliary β-subunits. J Neurophysiol 106:608–619. https://doi.org/10.1152/jn.00107.2011
Zhou Z, Gong Q, January CT (1999) Correction of defective protein trafficking of a mutant HERG potassium channel in human long QT syndrome. Pharmacological and temperature effects. J Biol Chem 274:31123–31126
Acknowledgments
We thank Brigitte Hoch for excellent technical assistance. This work was supported by the Core Facility Flow Cytometry, a Core Facility of the Interdisciplinary Center for Clinical Research (IZKF) Aachen within the Faculty of Medicine at RWTH Aachen University.
Funding
This study was funded in parts by the DFG (HA 6095/1-2 to RH, LA 2740/3-1 to AL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
ESM 1
(DOCX 380 kb)
Rights and permissions
About this article
Cite this article
Kaluza, L., Meents, J.E., Hampl, M. et al. Loss-of-function of Nav1.8/D1639N linked to human pain can be rescued by lidocaine. Pflugers Arch - Eur J Physiol 470, 1787–1801 (2018). https://doi.org/10.1007/s00424-018-2189-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2189-x