Abstract
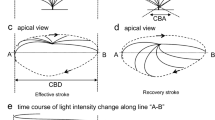
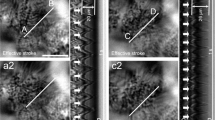
This study demonstrated that PDE1 (phosphodiesterase 1) existing in the ciliary beat frequency (CBF)-regulating metabolon regulates CBF in procaterol-stimulated lung airway ciliary cells of mouse. Procaterol (an β2-agonist) increased the ciliary bend angle (CBA) and CBF via cAMP accumulation in the ciliary cells of mice: interestingly, the time course of CBF increase was slower than that of CBA increase. However, IBMX (3-isobutyl-1-methylxanthine, an inhibitor of PDE) increased CBA and CBF in an identical time course. Lowering an intracellular Ca2+ concentration ([Ca2+]i) caused by switching to an EGTA-containing Ca2+-free solution from normal one elevated the procaterol-induced increasing rate of CBF. These observations suggest that Ca2+-dependent PDE1 controls cAMP-stimulated CBF increase. Either application of 8MmIBMX (8-methoxymethyl-IBMX, a selective PDE1 inhibitor), BAPTA-AM (an intracellular Ca2+ chelator), or calmidazolium (an inhibitior of calmodulin) alone increased CBA and CBF in the lung airway ciliary cells and increased cAMP contents in the isolated lung cells, and like IBMX, each application of the compound made the time courses of CBA and CBF increase stimulated by procaterol identical. The immunoelectron microscopic examinations revealed that PDE1A exists in the space between the nine doublet tubules ring and plasma membrane in the lung airway cilium, where the outer dynein arm (a molecular motor regulating CBF) functions. In conclusion, PDE1A is a key factor slowing the time course of the procaterol-induced increase in CBF via degradation of cAMP in the CBF-regulating metabolon of the mouse lung airway cilia.









Similar content being viewed by others
References
Afzelius BA (2004) Cilia-related diseases. J Pathol 204:470–477
Bender AT, Beavo JA (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharacol Rev 58:488–520
Brokaw CJ (1994) Control of flagellar bending: a new agenda based on dynein diversity. Cell Motil Cytoskeleton 28:199–204
Brokaw CJ, Kamiya R (1987) Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskeleton 8:68–75
Butcher RL, Collins WE, Fugo NW (1974) Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 94:1704–1708
Chilvers MA, Rutman A, O’Callaghan C (2003) Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J Allergy Clin Immunol 112:518–524
Delmotte P, Sanderson MJ (2006) Ciliary beat frequency is maintained at maximal rate in the small airways of mouse lung slices. Am J Respir Cell Mol Biol 35:110–117
Francis SH, Blount MA, Corbin JD (2011) Mammalian cyclic nucleotide pohosphodiesterase: molecular mechanism and physiological functions. Physiol Rev 91:651–690
Fuhrmann M, Jahn H-U, Seybold J, Neurohr C, Barnes PJ, Hippenstiel S, Kraemer HJ, Suttorp N (1999) Identification and function of cyclic nucleotide phosphodiesterase isoenzymes in airway epithelial cells. Am J Respir Cell Mol Biol 20:292–302
Gibbons IR, Rowe AJ (1965) Dynein: a protein with adenosine triohosphatase activity from cilia. Science 149:424–426
Hard R, Blaustein K, Scarcello L (1992) Reactivation of outer arm-depleted lung axonemes: evidence for functional differences between inner and outer dynein arms in situ. Cell Motil Cytoskeleton 21:199–209
Hayashi T, Kawakami M, Sasaki S, Katsumata T, Mori H, Yoshida H, Nakahari T (2005) ATP regulation of ciliary beat frequency in rat tracheal and distal airway epithelium. Exp Physiol 90:535–544
Jain R, Ray JM, Pan J-H, Brody SL (2012) Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol 46:446–453
Jourdan KB, Mason NA, Long L, Philips PG, Wilkins MR, Morrell NW (2001) Characterization of adenylyl cyclase isoforms in rat peripheral pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 280:L1359–L1369
Komatani-Tamiya N, Daikoku E, Takemura Y, Shimamoto C, Nakano T, Iwasaki Y, Kohda Y, Matsumura H, Marunaka Y, Nakahari T (2012) Procaterol-stimulated increases in ciliary bend amplitude and ciliary beat frequency in mouse bronchioles. Cell Physiol Biochem 29:511–522
Lefièvre L, de Lamirande E, Gagnon C (2002) Presence of cyclic nucleotide phosphodiesterases PDE1A, existing as a stable complex with calmodulin, and PDE3A in human spermatozoa. Biol Reprod 67:423–430
Li W-E, Chen W, Ma Y-F, Tuo Q-R, Luo X-J, Zhang T, Sai W-B, Liu J, Shen J, Liu Z-G, Zhen Y-M, Wang Y-X, Ji G, Liu Q-H (2012) Methods to measure and analyze ciliary beat activity: Ca2+ influx-mediated cilia mechanosensitivity. Pflügers Arch Eur J Physiol 464:671–680
Matsuzaki Y, Wu H, Nakano T, Nakahari T, Sano K (2015) ATP-association to intrabacterial nanotransportation system in Vibrio cholerae. Med Mol Morphol 48:225–234
Nakahari T, Nishimura A, Shimamoto C, Sakai A, Kuwabara H, Nakano T, Tanaka S, Kohda Y, Matsumura H, Mori H (2011) The regulation of ciliary beat frequency by ovarian steroids in the guinea pig Fallopian tube: interactions between oestradiol and progesterone. Biomed Res-Tokyo 32:321–328
Nakano T, Aoki H, Wu H, Fujioka Y, Nakazawa E, Sano K (2012) Fine visualization of filamentous structures in the bacterial cytoplasm. J Microbiol Methods 90:60–64
Salathe M (2007) Regulation of mammalian ciliary beating. Annu Rev Physiol 69:401–422
Salathe M, Bookman RJ (1999) Mode of Ca2+ action on ciliary beat frequency in single ovine airway epithelial cells. J Physiol 520:851–861
Shiima-Kinoshita C, Min K-Y, Hanafusa T, Mori H, Nakahari T (2004) β2-adrenergic regulation of ciliary beat frequency in rat bronchiolar epithelium: potentiation by isosmotic cell shrinkage. J Physiol 554:403–416
Smith RP, Shellard R, Dhillon DP, Winter J, Mehta A (1996) Asymmetric interactions between phosphorylation pathways regulating ciliary beat frequency in human nasal respiratory epithelium in vitro. J Physiol 496:883–889
Tanaka S, Hosogi S, Sawabe Y, Shimamoto C, Matumura H, Inui T, Marunaka Y, Nakahari T (2016) PPARα induced NOS1 phosphorylation via PI3K/Akt in guinea pig antral mucous cells: NO-enhancement in Ca2+-regulated exocytosis. Biomed Res-Tokyo 37(3):167–178
Turner MJ, Matthes E, Billet A, Ferguson AJ, Thomas DY, Randell SH, Ostrowski LE, Abbott-Banner K, Hanrahan JW (2016) The dual phosphodiesterase 3 and 4 inhibitor RPL554 stimulates CFTR and ciliary beating in primary cultures of bronchial epithelia. Am J Physiol Lung Cell Mol Physiol 310:L59–L70
Vasta V, Sonnenburg WK, Yan C, Soderling SH, Shimizu-Albergine M, Beavo JA (2005) Identification of a new valiant of PDE1A calmodulin-stimulated cyclic nucleotide phosphodiesterase expressed in mouse sperm. Biol Reprod 73:598–609
Wanner A, Salathe M, O’riordan TG (1996) Mucociliary clearance in the airways. Am J Respir Crit Care Med 154:1968–1902
Wood CR, Hard R, Hennessey TM (2007) Targeted gene disruption of dynein heavy chain 7 of Tetrahymena thermophila results in altered ciliary waveform and reduced swim speed. J Cell Sci 120:3075–3085
Wyatt TA, Forgèt MA, Sisson JH (2003) Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol 163:1157–1166
Acknowledgements
We thank the Osaka Medical College for giving us an opportunity to perform the experiments using the video microscope equipped with a high-speed camera. This work is partly supported by the contracted research fund from Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The procedures and protocols for the experiments were approved by the Committee for Animal Research of Kyoto Prefectural University of Medicine.
Rights and permissions
About this article
Cite this article
Kogiso, H., Hosogi, S., Ikeuchi, Y. et al. A low [Ca2+]i-induced enhancement of cAMP-activated ciliary beating by PDE1A inhibition in mouse airway cilia. Pflugers Arch - Eur J Physiol 469, 1215–1227 (2017). https://doi.org/10.1007/s00424-017-1988-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-1988-9