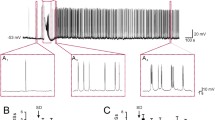
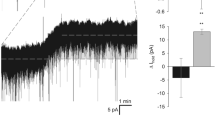
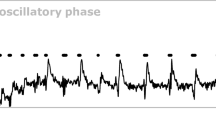
Abstract
GABAA receptor-mediated inhibition—which is due to Cl− and HCO3 − currents controlled by KCC2 and carbonic anhydrase activity, respectively—contributes to short- and long-lasting interictal events recorded from the CA3 region of hippocampus during application of 4-aminopyridine (4AP, 50 μM). Here, we employed field potential recordings in an in vitro brain slice preparation to establish the effects induced by the KCC2 blockers VU0240551 (10 μM) or bumetanide (50 μM) and by the carbonic anhydrase inhibitor acetazolamide (10 μM) on the two types of interictal events. We found that blocking KCC2 activity decreased the amplitude of the short-lasting events. In addition, this pharmacological procedure increased the interval of occurrence of the long-lasting events and reduced their amplitude. Blocking carbonic anhydrase activity with acetazolamide reduced the interval of occurrence and the duration of the short-lasting events while increasing their amplitude; acetazolamide also reduced the duration and amplitude of the long-lasting events. Finally, blocking either KCC2 or carbonic anhydrase activity increased the interval of occurrence of pharmacologically isolated synchronous GABAergic events and decreased their duration and amplitude. These data substantiate further the role of GABAA receptor-mediated signaling in driving neuronal populations toward hypersynchronous states presumably by increasing extracellular [K+].





Similar content being viewed by others
References
Avoli M, de Curtis M (2011) GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol 95:104–132
Avoli M, Methot M, Kawasaki H (1998) GABA-dependent generation of ectopic action potentials in the rat hippocampus. Eur J Neurosci 10:2714–2722
Barbarosie M, Avoli M (1997) CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci 17:9308–9314
Behr C, D’Antuono M, Hamidi S, Herrington R, Lévesque M, Salami P, Shiri Z, Kohling R, Avoli M (2014) Limbic networks and epileptiform synchronization: the view from the experimental side. Int Rev Neurobiol 114:63–87
Benini R, Avoli M (2005) Rat subicular networks gate hippocampal output activity in an in vitro model of limbic seizures. J Physiol 566:885–900
Blaesse P, Airaksinen MS, Rivera C, Kaila K (2009) Cation-chloride cotransporters and neuronal function. Neuron 61:820–838
Brückner C, Heinemann U (2000) Effects of standard anticonvulsant drugs on different patterns of epileptiform discharges induced by 4-aminopyridine in combined entorhinal cortex-hippocampal slices. Brain Res 859:15–20
Chamma I, Heubl M, Chevy Q, Renner M, Moutkine I, Eugène E, Poncer JC, Lévi S (2013) Activity-dependent regulation of the K/Cl transporter KCC2 membrane diffusion, clustering, and function in hippocampal neurons. J Neurosci 33:15488–15503
Chatrian GE, Bergamini L, Dondey M, Klass DW, Lennox-Buchthal M, Petersen I (1974) A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr Clin Neurophysiol 37:538–548
de Curtis M, Jefferys JGR, Avoli M (2012) Interictal epileptiform discharges in partial epilepsy: complex neurobiological mechanisms based on experimental and clinical evidence. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies [internet], 4th edn. National Center for Biotechnology Information (US), Bethesda
Delpire E, Days E, Lewis LM, Mi D, Kim K, Lindsley CW, Weaver CD (2009) Small-molecule screen identifies inhibitors of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci U S A 106:5383–5388
Delpire E, Baranczak A, Waterson AG, Kim K, Kett N, Morrison RD, Daniels JS, Weaver CD, Lindsley CW (2012) Further optimization of the K-Cl cotransporter KCC2 antagonist ML077: development of a highly selective and more potent in vitro probe. Bioorg Med Chem Lett 22:4532–4535
Dingledine R, Gjerstad L (1980) Reduced inhibition during epileptiform activity in the in vitro hippocampal slice. J Physiol 305:297–313
Dodgson SJ, Shank RP, Maryanoff BE (2000) Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia 41:S35–S39
Dulla CG, Frenguelli BG, Staley KJ, Masino SA (2009) Intracellular acidification causes adenosine release during states of hyperexcitability in the hippocampus. J Neurophysiol 102:1984–1993
Farrant M, Kaila K (2007) The cellular, molecular and ionic basis of GABA(A) receptor signaling. Prog Brain Res 160:59–87
Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, Perez-Sanchez J, Boudreau D, Wang B, Dumas L, Valade I, Bachand K, Jacob-Wagner M, Tardif C, Kianicka I, Isenring P, Attardo G, Coull JA, De Koninck Y (2013) Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med 11:1524–1528
Grover LM, Lambert NA, Schwartzkroin PA, Teyler TJ (1993) Role of HCO3- ions in depolarizing GABAA receptor-mediated responses in pyramidal cells of rat hippocampus. J Neurophysiol 69:1541–1555
Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C (2007) Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci 27:9866–9873
Jefferys JGR, Jiruska P, de Curtis M, Avoli M (2012) Limbic network synchronization and temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper's basic mechanisms of the epilepsies [internet], 4th edn. National Center for Biotechnology Information (US), Bethesda
Kaila K (1994) Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol 42:489–537
Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J (1997) Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci Off J Soc Neurosci 17:7662–7672
Löscher W, Puskarjov M, Kaila K (2013) Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology 69:62–74
Matsumoto H, Ajmone-Marsan C (1964) Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Exp Neurol 80:286–304
Michelson HB, Wong RK (1981) Excitatory synaptic responses mediated by GABAA receptors in the hippocampus. Science 253:1420–1423
Miles R, Blaesse P, Huberfeld G, Wittner L, Kaila K (2012) Chloride homeostasis and GABA signaling in temporal lobe epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper's basic mechanisms of the epilepsies [internet], 4th edn. National Center for Biotechnology Information (US), Bethesda
Morris ME, Obrocea GV, Avoli M (1996) Extracellular K+ accumulations and synchronous GABA-mediated potentials evoked by 4-aminopyridine in the adult rat hippocampus. Exp Brain Res 109:71–82
Perez Velazquez JL (2003) Bicarbonate-dependent depolarizing potentials in pyramidal cells and interneurons during epileptiform activity. Eur J Neurosci 18:1337–1342
Perreault P, Avoli M (1991) Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol 65:771–785
Perreault P, Avoli M (1992) 4-Aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci 12:104–115
Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipilä S, Payne JA, Minichiello L, Saarma M, Kaila K (2004) Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci 24:4683–4691
Rivera C, Voipio J, Kaila K (2005) Two developmental switches in GABAergic signalling: the K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol 562:27–36
Rutecki PA, Lebeda FJ, Johnston D (1987) 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol 57:1911–1924
Ruusuvuori E, Kaila K (2014) Carbonic anhydrases and brain pH in the control of neuronal excitability. Subcell Biochem 75:271–290
Ruusuvuori E, Li H, Huttu K, Palva JM, Smirnov S, Rivera C, Kaila K, Voipio J (2004) Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. J Neurosci Off J Soc Neurosci 24:2699–2707
Schwartzkroin PA, Prince DA (1980) Changes in excitatory and inhibitory synaptic potentials leading to epileptogenic activity. Brain Res 183:61–76
Staley KJ (2004) Role of the depolarizing GABA response in epilepsy. In: Binder DK, Scharfman HE (eds) Recent advances in epilepsy research. Kluwer Academic / Plenum Publishers, USA
Tolner EA, Hochman DW, Hassinen P, Otahal J, Gaily E, Haglund MM, Kubova H, Schuchmann S, Vanhatalo S, Kaila K (2011) Five percent CO2 is a potent, fast-acting inhalation anticonvulsant. Epilepsia 52:104–114
Traub RD, Wong RK (1982) Cellular mechanism of neuronal synchronization in epilepsy. Science 216:745–747
Viitanen T, Ruusuvuori E, Kaila K, Voipio J (2010) The K+-Cl− cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol 588:1527–1540
Voipio J, Kaila K (2000) GABAergic excitation and K(+)-mediated volume transmission in the hippocampus. Prog Brain Res 125:329–338
Voskyul RA, Albus H (1985) Spontaneous epileptiform discharges in hippocampal slices induced by 4-aminopyridine. Brain Res 342:54–66
Wahab A, Albus K, Heinemann U (2011) Age- and region-specific effects of anticonvulsants and bumetanide on 4-aminopyridine-induced seizure-like events in immature rat hippocampal-entorhinal cortex slices. Epilepsia 52:94–103
Zhu L, Polley N, Mathews GC, Delpire E (2008) NKCC1 and KCC2 prevent hyperexcitability in the mouse hippocampus. Epilepsy Res 79:201–212
Acknowledgments
This study was supported by the Canadian Institutes of Health Research (CIHR grants 8109 and 74609). We thank Dr. M. Levesque, Ms. R. Herrington and Ms. P. Salami for helping with the recording procedures and data analysis. We also thank Dr. Yves De Koninck for generously providing us with CLP275.
Conflicts of interest
None of the authors has any conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamidi, S., D’Antuono, M. & Avoli, M. On the contribution of KCC2 and carbonic anhydrase to two types of in vitro interictal discharge. Pflugers Arch - Eur J Physiol 467, 2325–2335 (2015). https://doi.org/10.1007/s00424-015-1686-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-015-1686-4