Abstract
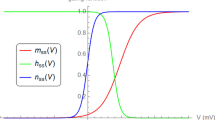
Rapidly adapting (RA) currents expressed in dorsal root ganglia somatosensory neurons reduce their amplitude in response to prolonged and/or repeated mechanical stimulation. Both inactivation of mechanotransducer channels and adaptation of the force acting on the channels have been suggested to independently decrease RA currents. However, these two mechanisms have similar kinetics and dependence on calcium and voltage. These experimental findings suggest that a single mechanism might underlie both. We constructed a simple Hodgkin–Huxley-type model with a single gating variable driving both inactivation and adaptation to test this hypothesis. Predictions of the model successfully describe key features of mechanical activation as well as inactivation, adaptation, and recovery. The model thus supports the possibility of a single mechanism driving inactivation and adaptation in RA currents. On its own, the model can be integrated into higher-order models of touch receptors because of its accurate simulation of RA currents.







Similar content being viewed by others
References
Drew LJ, Wood JN, Cesare P (2002) Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J. Neurosci 22:RC228:(1–5)
Hu J, Lewin GR (2006) Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J Physiol 577(Pt 3):815–828. doi:10.1113/jphysiol.2006.117648
Hao J, Delmas P (2010) Multiple desensitization mechanisms of mechanotransducer channels shape firing of mechanosensory neurons. J Neurosci 30(40):13384–13395. doi:10.1523/JNEUROSCI.2926-10.2010
Rugiero F, Drew LJ, Wood JN (2010) Kinetic properties of mechanically activated currents in spinal sensory neurons. J Physiol 588(Pt 2):301–314. doi:10.1113/jphysiol.2009.182360
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330(6000):55–60. doi:10.1126/science.1193270
Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A (2014) Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516(7526):121–125
Hao J, Bonnet C, Amsalem M, Ruel J, Delmas P (2015) Transduction and encoding sensory information by skin mechanoreceptors. Eur J Physiol 467(1):109–119
Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN (2004) Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol 556(Pt 3):691–710. doi:10.1113/jphysiol.2003.058693
Eatock RA, Corey DP, Hudspeth AJ (1987) Adaptation of mechanoelectrical transduction in hair cells of the bullfrog’s sacculus. J Neurosci 7(9):2821–2836
Eatock RA (2000) Adaptation in hair cells. Annu Rev Neurosci 23:285–314. doi:10.1146/annurev.neuro.23.1.285
Gillespie PG, Müller U (2009) Mechanotransduction by Hair Cells. Cell 139(1):33–44. doi:10.1016/j.cell.2009.09.010
Armstrong CM (1981) Sodium channels and gating currents. Physiol Rev 61(3):644–683
Kellenberger S, Scheuer T, Catterall WA (1996) Movement of the Na+ channel inactivation gate during inactivation. J Biol Chem 271(48):30971–30979
Shin KS, Maertens C, Proenza C, Rothberg BS, Yellen G (2004) Inactivation in HCN channels results from reclosure of the activation gate: desensitization to voltage. Neuron 41(5):737–744
Akitake B, Anishkin A, Sukharev S (2005) The dashpot mechanism of stretch-dependent gating in MscS. J Gen Physiol 125:143
Belyy V, Anishkin A, Kamaraju K, Liu N, Sukharev S (2010) The tension-transmitting’clutch’ in the mechanosensitive channel MscS. Nature Structural and Molecular Biology 17(4):451
Howard J, Roberts WM, Hudspeth AJ (1988) Mechanoelectrical transduction by hair cells. Annu Rev Biophys Biophys Chem 17:99–124. doi:10.1146/annurev.bb.17.060188.000531
Hao J, Padilla F, Dandonneau M, Lavebratt C, Lesage F, Noël J, Delmas P (2013) Kv1. 1 channels act as mechanical brake in the senses of touch and pain. Neuron 77(5):899–914. doi:10.1016/j.neuron.2012.12.035
Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A (2012) Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483(7388):176–181. doi:10.1038/nature10812
Katta S, Krieg M, Goodman MB (2015) Feeling force: physical and physiological principles enabling sensory mechanotransduction. Annu Rev Cell Dev Biol 31:347–371
Gottlieb PA, Bae C, Sachs F (2012) Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels (Austin) 6(4):282–289. doi:10.4161/chan.21064
Coste B, Houge G, Murray MF, Stitzield N, Bandelle M, Giovanni MA, Philippakis A, Hoischen A, Riemer G, Steen U, Steen VM, Mathur J, Cox J, Lebo M, Rehm H, Weiss ST, Wood JN, Maas RL, Sunyaev SR, Patapoutian A (2013) Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proc Natl Acad Sci USA 110:4667–4672
Ranade SS, Syeda R, Patapoutian A (2015) Mechanically activated ion channels. Neuron 87:1162–1179
Hu J, Chiang LY, Koch M, Lewin GR (2010) Evidence for a protein tether involved in somatic touch. EMBO J 29(4):855–867. doi:10.1038/emboj.2009.398
Kung C (2005) A possible unifying principle for mechanosensation. Nature 436(7051):647–654. doi:10.1038/nature03896
Hardie RC, Franze K (2012) Photomechanical responses in Drosophila photoreceptors. Science 338(6104):260–263. doi:10.1126/science.1222376
Sukharev S, Sachs F (2012) Molecular force transduction by ion channels–diversity and unifying principles. J Cell Sci 125:3075–3083
Vodlak T, Vidrih Z, Pirih P, Škorjanc A, Prešern J, Rodič T (2014) Functional microanatomical model of Meissner corpuscl. In: Haptics: Neuroscience, Devices, Modeling, and Applications (Springer), pp. 377–384
Acknowledgments
We are grateful to Drs Patrick Delmas and Jizhé Hao for sending us some of the experimental data and to Eric James McDermott for proofreading.
Author information
Authors and Affiliations
Corresponding author
Additional information
TR was supported by EU FP7 NanoBioTouch. JB was supported by BMBF Bernstein Award Computational Neuroscience 01GQ0802. JP received support from the Slovenian Research Agency, project ARRS-Z3-4061, and AŠ received support from the Slovenian Research Agency, project ARRS-Z1-4288.
Rights and permissions
About this article
Cite this article
Prešern, J., Škorjanc, A., Rodič, T. et al. A single mechanism driving both inactivation and adaptation in rapidly adapting currents of DRG neurons?. Biol Cybern 110, 393–401 (2016). https://doi.org/10.1007/s00422-016-0693-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-016-0693-7